If you change the Pressure to 4 bar at 700 K, how will the equilibrium composition of H2 change (relative to part (a))? Explain your answer.
The “water-gas” shift reaction is catalyzed by an iron oxide/chromium oxide catalyst at 700 K and 2 bar.
H2O + CO ? CO2 +H2
System pressure has no effect on the equilibrium composition of hydrogen according to the equilibrium criterion relationship derived. Since ??v_i =0, the (P^0/P) term becomes 1.
However, if the pressure became high enough that ideal gas behavior cannot be assumed, the fugacity coefficient terms must be accounted for in the equilibrium relationship:
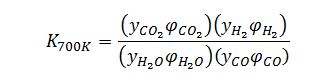
So the equilibrium composition and therefore the mole fraction of hydrogen could be influenced in that sense.
You might also like to view...
Draw a flowchart to calculate the sum of the areas of circles whose radii are 2, 3, 4, and 5 cm.
What will be an ideal response?
The best material to use for special protective clothing is ____.
A. polyester B. 100% cotton C. 100% wool D. leather
Having to add water to a low-maintenance battery can be an indication of a higher-than-specified charging voltage.
Answer the following statement true (T) or false (F)
Describe the purpose of isometric and oblique sketches.
What will be an ideal response?