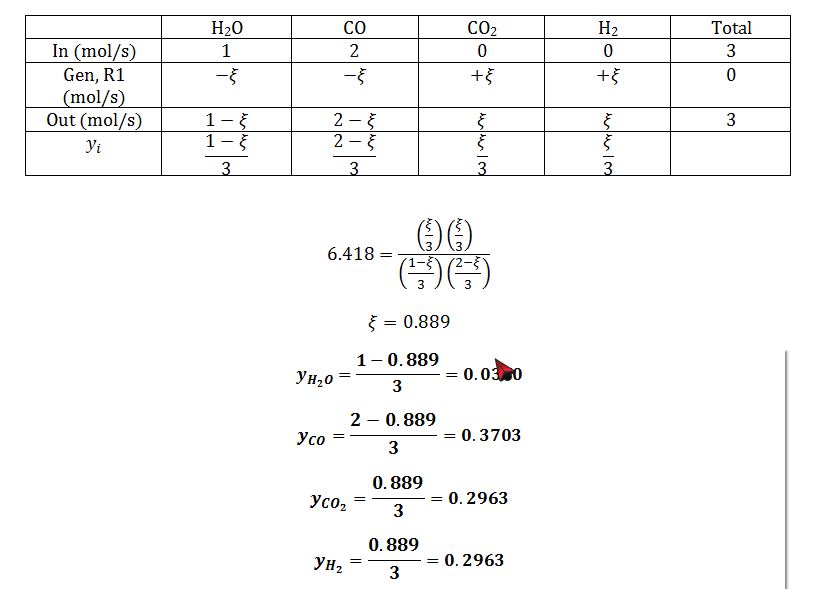
What is the equilibrium composition of this system at that T and P if you start with a 1 mole of H2O and 2 moles of CO? Assume the heat of reaction is not a function of temperature.
The “water-gas” shift reaction is catalyzed by an iron oxide/chromium oxide catalyst at 700 K and 2 bar.
H2O + CO ? CO2 +H2
Apply short-cut van`t Hoff equation to find ??G_700K^0 since heat of reaction is not a function of temperature:
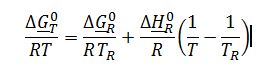
Where ??G_T^0 is the Gibbs free energy for reaction @ 700K, T=700 K, T_R=298 K, ??H_R^0=?????H_(f,products)^0-? ?????H_(f,reactants)^0 ? and ??G_R^0=?????G_(f,products)^0-? ?????G_(f,reactants)^0 ?. Values for ??H_f^0 and ??G_f^0 are obtained from Appendix C-2:
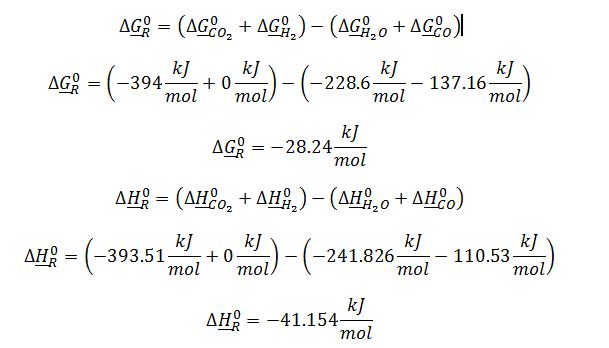
Substitute known terms into van`t Hoff equation:
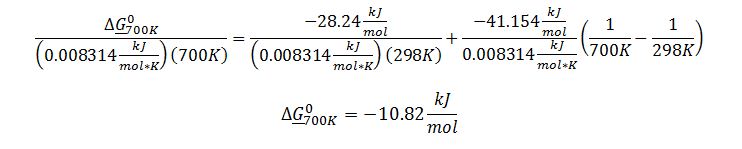
Apply definition of equilibrium constant:
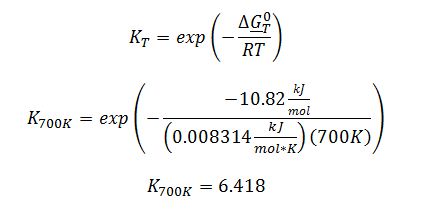
Apply equilibrium criterion for gas phase reaction:
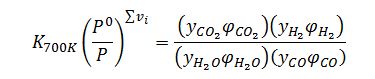
Where ??v_i =0. Assume all ?_i=1 since pressure is low enough to assume ideal gas behavior:
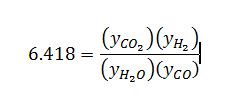
Apply stoichiometric table to get mole fractions in terms of extent of reaction:
You might also like to view...
In a CAN network, if a module fails to "check in" with the other modules:
A. The module will be shut down B. A trouble code is set C. The vehicle will not start. D. None of the above
What is the meaning of the term anneal?
What will be an ideal response?
Breeding birds for broilers are selected for rapid growth and feed efficiency
Indicate whether the statement is true or false
? Identify and state the historical significance of the New Deal.
What will be an ideal response?