Solve the problem.The volume V (in liters) of 1 mole of a gas is related to its temperature T (in Kelvin) and pressure P (in atmospheres) by van der Waals' equation 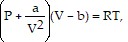 where the constant R is 0.08207. For krypton,
where the constant R is 0.08207. For krypton, 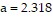 and
and 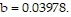 Use Newton's method to find the volume V of 1 mole of krypton if
Use Newton's method to find the volume V of 1 mole of krypton if 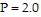 atmospheres and
atmospheres and
src="https://sciemce.com/media/4/ppg__tttt0604191018__f1q68g5.jpg" alt="" style="vertical-align: -4.0px;" /> Round your answer to two decimal places.
A. 17.61 L
B. 13.50 L
C. 15.49 L
D. 12.53 L
Answer: B
Mathematics
src="https://sciemce.com/media/4/ppg__tttt0604191018__f1q68g5.jpg" alt="" style="vertical-align: -4.0px;" /> Round your answer to two decimal places.
A. 17.61 L
B. 13.50 L
C. 15.49 L
D. 12.53 L
Answer: B
Mathematics
You might also like to view...
Compute the gradient of the function at the given point.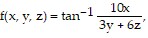
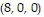
A. ?f = 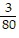 j -
j - 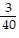 k
k
B. ?f = - 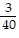 j +
j + 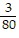 k
k
C. ?f = - 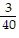 j -
j - 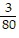 k
k
D. ?f = - 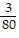 j -
j -  k
k
Mathematics
Write a chain rule formula for the following derivative.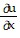 for u = f(p, q); p = g(x, y, z), q = h(x, y, z)
for u = f(p, q); p = g(x, y, z), q = h(x, y, z)
A. 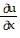 =
= 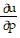
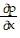 +
+ 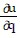
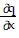
B. 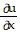 =
= 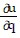
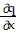
C. 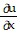 =
= 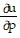
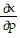 +
+ 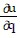
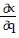
D. 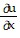 =
= 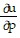
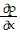 +
+ 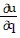
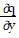 +
+ 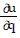
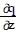
Mathematics
Simplify the expression.j16
A. j B. 1 C. -j D. -1
Mathematics
Provide an appropriate response. Find the related angle for 245°.
A. 65° B. 180° C. 25° D. 0°
Mathematics