Solve the problem.The pressure of oxygen gas in a closed container can be described approximately by the Dieterici equation P =  e-0.0006/T,where T is the Kelvin temperature of the sample and v is the volume in liters.If
e-0.0006/T,where T is the Kelvin temperature of the sample and v is the volume in liters.If 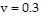 and
and 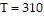 , in what "direction" should we change (v, T) to make the pressure increase as rapidly as possible?
, in what "direction" should we change (v, T) to make the pressure increase as rapidly as possible?
A. In the direction 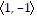
B. In the direction 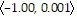
C. In the direction 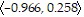
D. In the direction 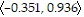
Answer: B
Mathematics
You might also like to view...
Evaluate the integral. dx
dx
A. x + 1 + C
+ 1 + C
B. 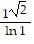
C. 1 + 1 - 1
+ 1 - 1
D. 1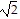 + 1
+ 1
Mathematics
Multiply and simplify.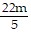 ?
? 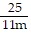
A. 5m
B. 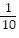
C. 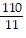
D. 10
Mathematics
Use synthetic division to find the quotient and the remainder.x5 + x2 + 2 is divided by x + 3
A. x4 - 2; remainder 8 B. x4 - 3x3 + 10x2 - 30x + 90; remainder -268 C. x4 - 3x3 + 9x2 - 26x + 78; remainder -232 D. x4 - 2x2; remainder 8
Mathematics
Determine whether the integral is convergent or divergent.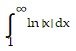
A. Divergent B. Convergent
Mathematics