Linear density in a given crystallogrphic direction represents the fraction of a line length that is occupied by atoms. Similarly, planar density is the fraction of a crystallographic plane occupied by atsom. The fraction of the volume occupied in a unit cell, on the other hand, is called the atomic packing factor. The latter should not be confused with bulk densirt, which represents weight per unit volume.
(a) Calculate the linear density in the [100], [110], and [111]directions in body?ccentered cubic(bcc) and face?centered cubic (fcc) structures.
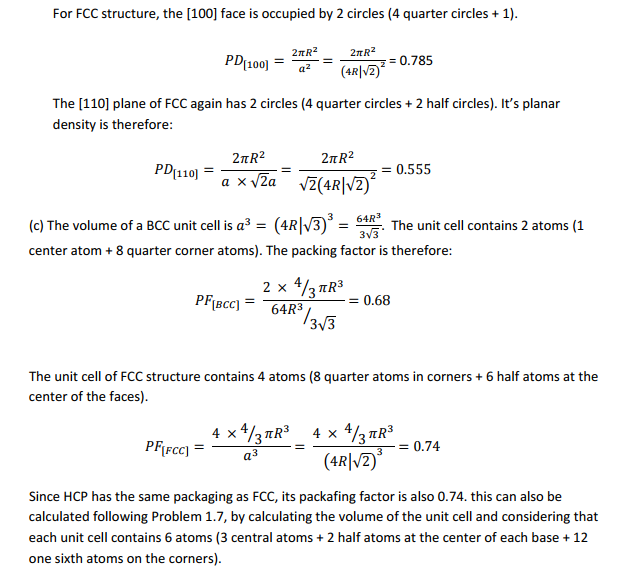
(b) Calculate planar densities in (100), (110), and (111) planes in bcc and fcc structires.
(c) Show that atomic packing factors for bcc, fcc, and hexagonal close?packed (hcp) structuresare 0.68, 0.74, and 0.74, respectively.

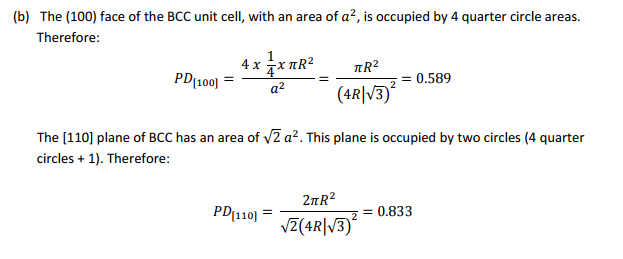
You might also like to view...
The unijunction transistor is made by combining ____ layers of semiconductor material.
A. two B. three C. four D. five
Heat that goes into matter and results in a temperature increase is called ____________________.
Fill in the blank(s) with the appropriate word(s).
Which of the following is NOT an example of an actuator?
A. joysticks B. stepper motors C. solenoids D. proportioning solenoids
The edges of a dovetail are "broken", preventing:
A. A radius B. A fillet edge C. A sharp corner D. A flat