One mole of an ideal gas with 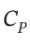 = (7/2)R and
= (7/2)R and 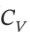 = (5/2)R expands from
= (5/2)R expands from 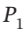 = 8 bar and
= 8 bar and 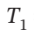 = 600 K to
= 600 K to 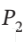 = 1 bar by each of the following paths:
= 1 bar by each of the following paths:
What will be an ideal response?
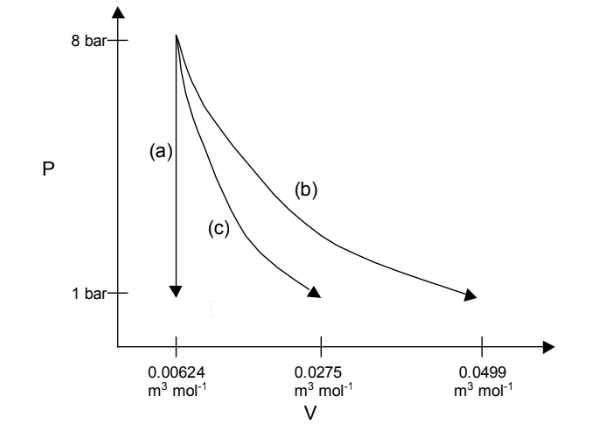
(a) Constant volume;
(b) Constant temperature;
(c) Adiabatically.
Assuming mechanical reversibility, calculate W, Q, ?U, and ?H for each process. Sketch each path on a single PV diagram.
a. Constant volume expansion from 8 bar and 600 K to 1 bar. Since PV/T is constant for an ideal gas, when P decreases by a factor of 8, T will also decrease by a factor of 8 to maintain constant volume, so in this case the final temperature is 600/8 = 75 K. As for any constant volume process, the work done is zero (W = 0). The heat transfer required is

The change in internal energy is ?U = Q = -10910 J 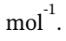
The change in enthalpy is
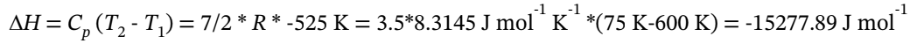
b. For isothermal expansion, we know that ?H = ?U = 0.
The heat flow required is
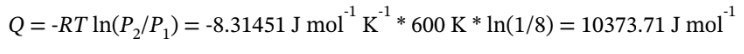
The work done is
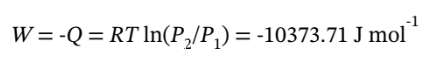
c. For adiabatic expansion, we have
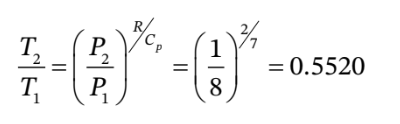
So we have  = 0.5520*600 K = 331 K
= 0.5520*600 K = 331 K
Then 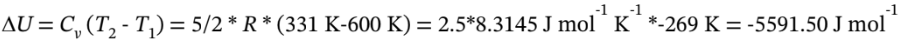
And 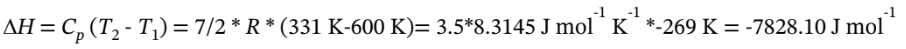
By definition, Q = 0.
Finally, W = ?U = -5591.50 J 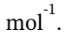
On a PV diagram, the three paths look like this:
You might also like to view...
Sudangrass and ____________________-sudangrass hybrids should never be fed to horses. They contain compounds that can cause muscle weakness, urinary problems, and death in severe cases.
Fill in the blank(s) with the appropriate word(s).
The part that can MOST-Likely be used during transmission assembly to make reinstallation of the countershaft much easier is:
A. bearings B. countergear C. helical gear D. dummy shaft
The force that causes electrons to move from atom to atom in a conductor is called __________.
a. amperage b. ohms c. voltage d. watts
Each side of the uct elbow is called a "cheek".
Answer the following statement true (T) or false (F)