The mass of an oxygen molecule is 16 times that of a hydrogen molecule. At room temperature, the ratio of the rms speed of an oxygen molecule to that of a hydrogen molecule is:
A. 16
B. 4
C. 1
D. 1/4
E. 1/16
Answer: D. 1/4
You might also like to view...
The size of Olympus Mons suggests that the crust of Mars is very thick
Indicate whether the statement is true or false
In the figure, a 1.6-kg weight swings in a vertical circle at the end of a string having negligible weight. The string is 2 m long. If the weight is released with zero initial velocity from a horizontal position, its angular momentum (in kg?m2/s) at the lowest point of its path relative to the center of the circle is approximately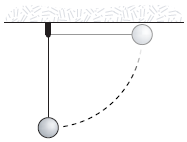
A. 40 B. 10 C. 30 D. 20 E. 50
The mass of a high speed train is 4.5×105kg, and it is traveling forward at a velocity of 8.3×101m/s. Given that momentum equals mass times velocity, determine the values of m and n when the momentum of the train (in kg?m/s) is written in scientific notation.?
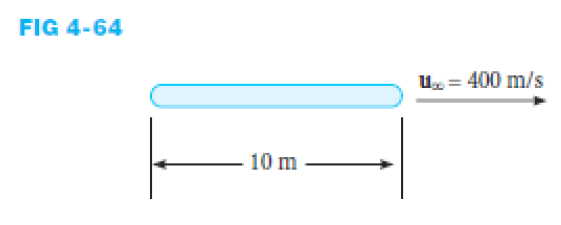
A wing of a particular airplane is 10 m wide. During flight conditions air at 80 kPa and - 10ºC passes around the wing at 400 m/s. If the wing surface temperature is 80ºC determine: a) how much of the boundary layer is turbulent if the critical Reynolds number is taken as 5 x 10^5, b) the average convective heat transfer coefficient, and c) the heat transfer per unit area.