Determine the total energy of water vapor with a mass of 2.50 kg, a specific internal energy of 2780 kJ/kg, traveling at a velocity of 56.0 m/s at a height of 3.50 m.
Given: m = 2.50 kg ; z = 3.50 m; V = 56.0 m/s; u = 2780 kJ/kg
Assume: g = 9.81 m/s2
What will be an ideal response?
E = mu + ½ mV2 + mgz
= (2.50 kg)[2780 kJ/kg + ½ (56.0 m/s)2/(1000 J/kJ) + (9.81 m/s2)(3.50 m)/(1000 J/kJ)]
E = 6950 kJ (6954 kJ)
You might also like to view...
When the market price increases to $8 per pound, which of the following statements is true?
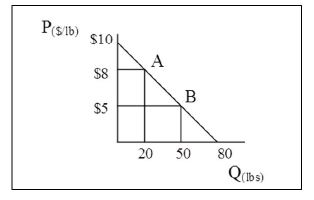
A) Consumers are better off by $105.
B) Consumers are better off by $20.
C) Consumers are worse off by $20.
D) Consumers are worse off by $105.
Animals raised in disease-free environments cost less and pose less risk
Indicate whether the statement is true or false
Explain how spontaneous combustion is caused by oily rags.
What will be an ideal response?
The center port on a four-valve manifold is marked VAC and is used for __________.
A. evacuation purposes B. refrigerant recovery C. system charging D. system leak testing