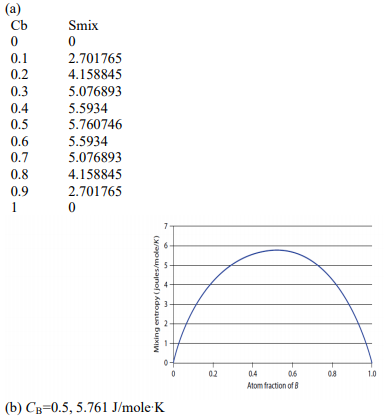
Use a spreadsheet
(a) Use a spreadsheet program such as Microsoft Excel or write a
computer program to calculate and then plot the entropy change relative to the pure separated elements for mixing 1 mole total of A-type and B-type atoms that form an ideal solution. Make this calculation in increments of 0.1 atom fraction change.
(b) What chemical composition has the largest entropy of mixing, and what is this value?
You might also like to view...
Why is there an upper end in the main sequence of the H-R diagram?
What will be an ideal response?
Elliptical galaxies contain more gas, dust, and young stars than do Sa galaxies
a. True b. False Indicate whether the statement is true or false
What evidence suggests that Mercury contracted within about a billion years after it formed?
Fill in the blank(s) with the appropriate word(s).
The same object in different inertial reference systems:
a. will always have the same kinetic energy, regardless of the reference system. b. can have different amounts of kinetic energy viewed from each inertial reference system. c. cannot be studied from two different reference systems. d. can have different amounts of gravitational potential energy in different reference systems, but will always have the same amount of kinetic energy. e. sometimes conserves energy and sometimes does not, depending on inertial reference system.