It can be shown that the change in entropy in heating or cooling a sample is given by the relation  = mc ln
= mc ln 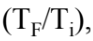 where m is the mass of the sample, c is the specific heat of the sample, and
where m is the mass of the sample, c is the specific heat of the sample, and 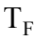 and
and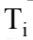 are the final and initial temperatures respectively. What is the change in entropy when 20.0 grams of aluminum with a specific heat of 0.9 J/gm K is heated from 10.0
are the final and initial temperatures respectively. What is the change in entropy when 20.0 grams of aluminum with a specific heat of 0.9 J/gm K is heated from 10.0 C 60.0
C 60.0
src="https://sciemce.com/media/4/4efdbe7f0e8622ad72.png" class="w-image" />C?
A. +2.93 J/K
B. +2.50 J/K
C. 0 J/K
D. -2.93 J/K
A. +2.93 J/K
Physics & Space Science
src="https://sciemce.com/media/4/4efdbe7f0e8622ad72.png" class="w-image" />C?
A. +2.93 J/K
B. +2.50 J/K
C. 0 J/K
D. -2.93 J/K
A. +2.93 J/K
You might also like to view...
The Moon must be at least _____ years old
A) 4.48 billion B) 5.62 billion C) 6.28 billion D) 7.14 billion
Kirchhoff's Rules: Determine the current in the 8.0-? resistor for the circuit shown in the figure. Assume that the batteries are ideal and that all numbers are accurate to two significant figures.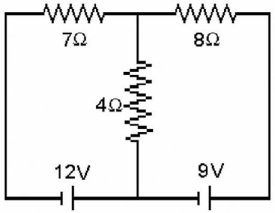
A. 0.28 A B. 1.3 A C. 1.6 A D. 2.1 A
A friend is running clockwise at a constant speed on a circular track. At the instant your friend is running due east, your friend is due west of you. What is the direction of your friend’s angular momentum around you at that instant? Choose from the possible directions presented in problem C7T.3, remembering that this is a top view, with A corresponding to north, C to east, and so on. 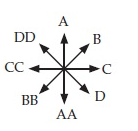
A. A B. B C. C D. D E. Upward F. Downward T. (The vector is zero)
High mass stars are much less common than low mass stars because they
A) are born in much smaller numbers B) have much shorter lifetimes C) have much longer lifetimes D) two of these