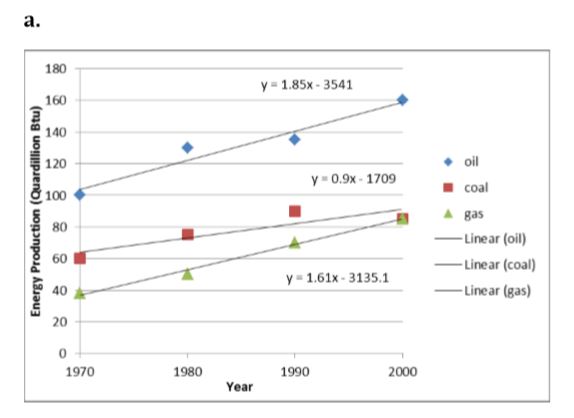
World energy production by source is shown in Figure 6.51. Using Excel, plot the data for crude oil, coal, and natural gas for each decade: 1970, 1980, 1990, and 2000. Establish a linear trend line through your graph for each fuel source. Also add a data set for the summation of each source to approximate world energy use of fossil fuels.
a. Forecast and extend the x-axis time and the trend line backwards to 1850 and forward to 2050. Show this trend line on a graph.
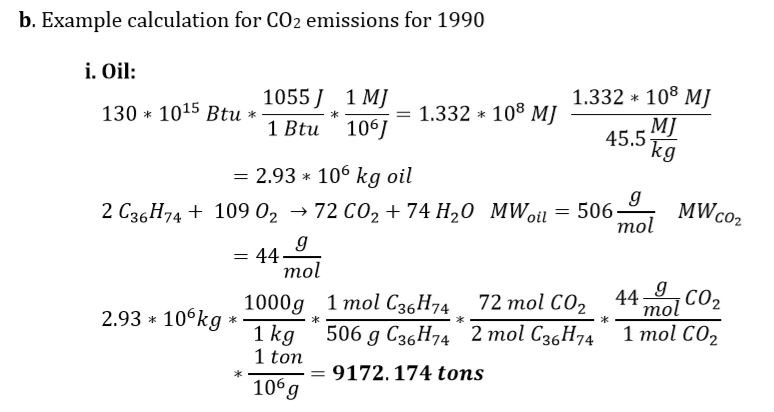
b. Show on a sheet of paper an example calculation for CO2 emissions for the year 1990 for:
i) Oil
ii) Coal
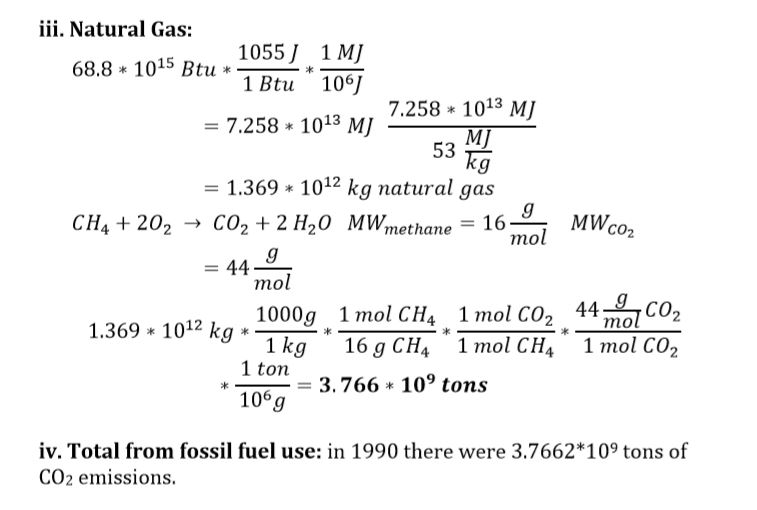
iii) Natural gas
iv) All CO2 emissions for 1990 from fossil fuel use
What will be an ideal response?
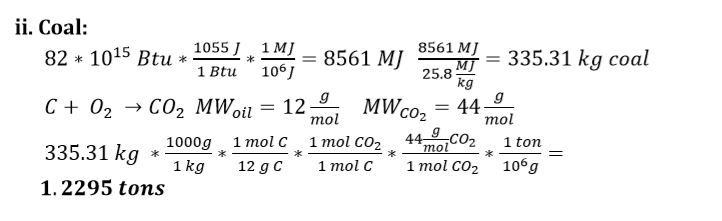
You might also like to view...
Color-contrast penetrants contain a colored dye that is often _____ in color.? ?
A. blue? B. green? C. red? D. yellow?
A) Find the molar flow rate of the liquid product. B) Find the temperature of the recycled vapor that exits the countercurrent heat exchanger.
A compound’s boiling point at P=0.5 bar is 100 K. The Linde liquefaction process is used to produce this compound in the form of saturated liquid at 100 K. The steady state process is described as follows: • The fresh feed joins a recycle stream. The mixed stream has a flow rate of 200 mol/min. • A series of compressors and heat exchangers converts the stream into a supercritical fluid at P=100 bar and T=160 K. At these conditions, the compound has a molar enthalpy of 3000 J/mol. • The supercritical fluid enters a counter-current heat exchanger that decreases its temperature to 140 K, while maintaining the pressure of 100 bar. • The 200 mol/min of supercritical fluid enters a flash chamber in which the pressure is maintained at 0.5 bar. • The liquid product leaving the chamber is saturated liquid at 100 K and has a molar enthalpy of 0 J/mol. • The vapor leaving the chamber has a molar enthalpy of 2500 J/mol. It is used as the coolant in the counter current heat exchanger, and is then recycled and mixed with the fresh feed. The compound in the vapor phase behaves as an ideal gas with CP*=6R at all pressures equal to or below 1 bar, and it has CP=8R in the supercritical state at P=100 bar.
Which of the following statements is most accurate?
A) Core systems must adjust to accommodate variables such as wind effects. B) Perimeter systems only need to accommodate loads such as electrical equipment and lighting. C) Core systems are subject to a wider range of variables than perimeter systems. D) Perimeter systems accommodate many of the loads of the core system and loads caused by weather.
A low-pass filter is one that _____.
A. increases opposition dramatically after the frequency reaches the cut-off frequency B. passes frequencies in a very narrow frequency range C. passes a range of frequencies while rejecting frequencies on either side D. rejects a range of frequencies while passing frequencies on either side