Sodium metal is paramagnetic with a dimensionless magnetic susceptibility (?) of 8.48×10-6 . The paramagnetism of sodium metal is due to the rotation of some of the spins of the metal’s free electrons into the direction of the applied magnetic field. The applied magnetic field is equal to 1×105 A/m. Sodium metal is BCC with a lattice parameter of 0.428×10-9 m, and sodium is from Group 1A of the periodic table.
(a) What is the intensity of magnetization or the dipole moment per unit volume?
(b) For sodium, what is the number of atoms per unit volume, and what is the number of free electrons per unit volume?
(c) What is the dipole moment per sodium atom?
(d) Assuming that all of the magnetic susceptibility is due to the orientation of free electron spins, what is the net number of free electron spins per atom oriented in the direction of the applied magnetic field?

You might also like to view...
Nuclear Reactions: The equation shows a fission reaction: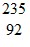 U +
U + 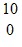 n ?
n ?  Nb + X + 4
Nb + X + 4 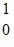 nWhat is the missing term X in the equation?
nWhat is the missing term X in the equation?
A. 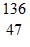 Ag
Ag
B. 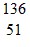 Sb
Sb
C. 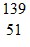 Sb
Sb
D. 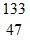 Ag
Ag
E. 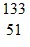 Sb
Sb
What is proper motion? How is it related to transverse velocity?
What will be an ideal response?
Why are so few molecules observed in visible light? Where are most observed, and why?
What will be an ideal response?
Consider a situation where you exert a force F on a crate of mass m, moving it at a speed v a distance d across a floor in a time interval t.The kinetic energy of the crate is __________
Fill in the blank(s) with correct word