Nitrogen has a specific volume of 0.25 m3/kg. Using both the ideal gas law and van der Waals equation, tabulate the temperature of the nitrogen for pressures varying between 250 kPa and 2500 kPa, and plot the difference between the two values as a function of pressure.
Given: v = 0.25 m3/kg, N2
What will be an ideal response?
For N2, R = 0.2968 kJ/kg-K
From the ideal gas law: T = Pv/R
For van der Waals Equation, we need the constants a and b:
First, for N2, Tc = 126.2 K, Pc = 3390 kPa
Then, a = 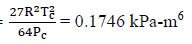
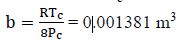
From van der Waals equation: 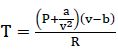
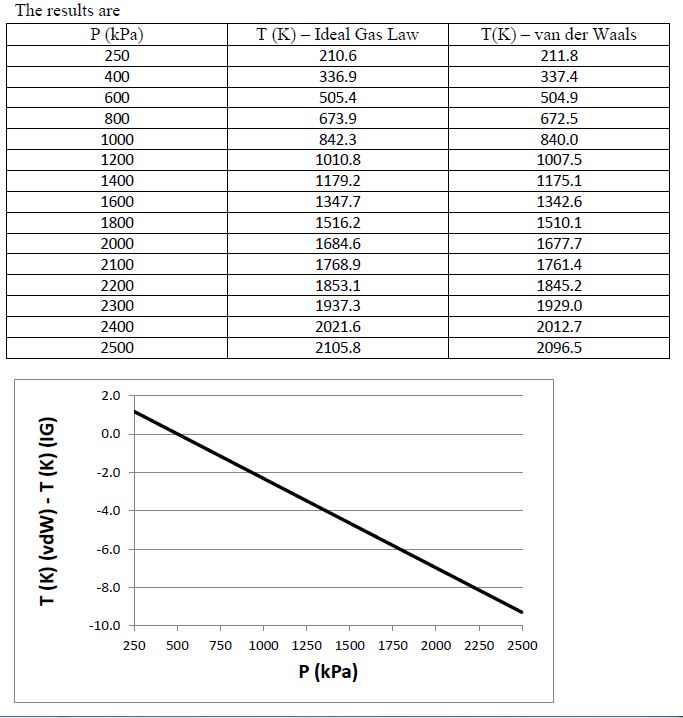
You might also like to view...
Technician A says that only offshore engines are permitted to use port-helix metering fuel systems on EPA MY 2010 compliant and later engines. Technician B says that port-helix metering injection pumps can be used on current diesel engines when the peak power rating is less than 70 BHP (52 kW). Who is correct??
A. ?Technician A only B. ?Technician B only C. ?Both A and B D. ?Neither A nor B
Additional vitamin ____________________ in the feed the animal receives prior to slaughter can help the meat retain optimal color
Fill in the blank(s) with correct word
Answer the following statement(s) true (T) or false (F)
1. A bonding jumper for a supplemental grounding electrode is required to be not smaller than 14 AWG. 2. The NEC defines the term ground as “the earth”. 3. A chassis ground provides safety from ground faults and possible short circuits. 4. The grounding electrode conductor is typically green in color. 5. A metal gas pipe is a suitable ground but only for communication equipment operating at 30V or less.
A liquid-vapor equilibrium mixture is confined in a closed system with a total volume of 1 m3. The temperature of the mixture is 350 K and the pressure is 150 kPa. The liquid has a molar volume of 8 × 10-5 m3/mol and the vapor can be modeled as an ideal gas. If there are 100 total moles, what is the number of moles in the liquid phase?
A. 51.3 mol B. 48.7 mol C. -51.3 mol D. 0.414 mol E. Only 1 degree of freedom exists in the system, but two properties are specified (T and P), so the problem is invalid/void/impossible.