Energy Levels: The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength ? is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels?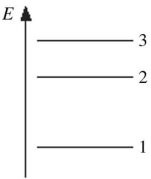
A. both ?/2 and ?/3
B. only ?/2
C. both 2? and 3?
D. only 2?
Answer: A
You might also like to view...
The white-hot sparks from a 4th-of-July-type sparkler that strike your skin transfer
A) little energy to you in spite of their high temperature. B) little energy to you due to their low temperature. C) much energy, but at a low temperature. D) none of the above
In general, the narrower the spectral line of a star:
A) the hotter the star is. B) the bigger the star is. C) the denser the star is. D) the smaller the star is. E) the cooler the star is.
On the equator, days and nights are always 12 hours long, no matter what time of year
Indicate whether the statement is true or false
Molecules of which monomer could, by themselves, form a condensation polymer?
a. HO–CH2–CH2–NH2 b. H2N–CH2–COOH c. HO–CH2–CH2–OH d. HOOC–COOH