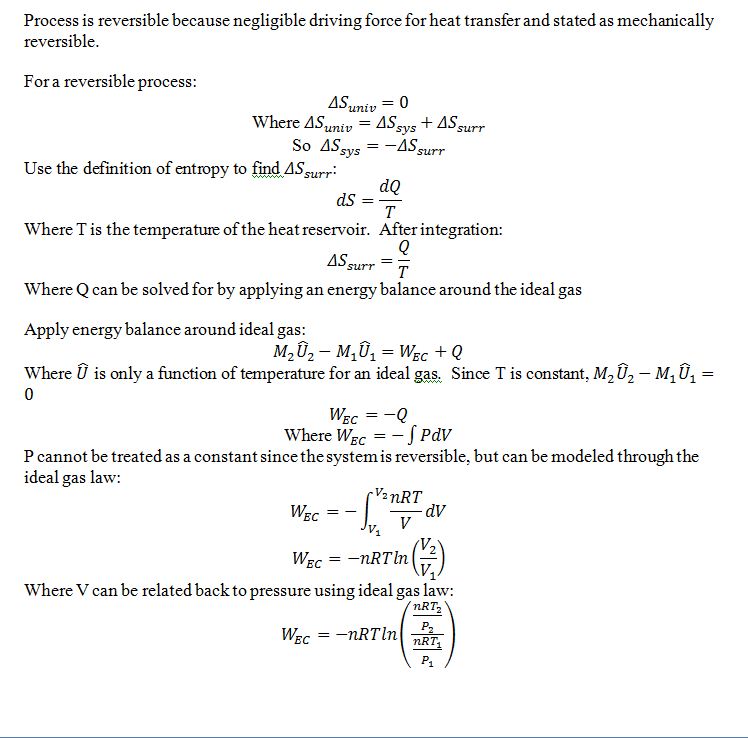
One-thousand moles of an ideal gas is compressed isothermally at 400 K from 100 kPa to 1000 kPa in a piston-cylinder arrangement. Calculate the entropy change of the gas, the entropy change of the surroundings and the total entropy change of the process for the following two cases:
(a) The process is mechanically reversible and the surroundings consist of a heat reservoir at 400 K.
(b) The process is mechanically reversible and the surroundings consist of a heat reservoir at 300 K.

You might also like to view...
Authorities recommend that dogs live in nursing homes with residents
Indicate whether the statement is true or false
Give three examples of some common health symptoms caused by poor indoor air quality.
What will be an ideal response?
With the FLATSHOT command you can quickly obtain projected views that use different properties for visible and occulted lines
Indicate whether the statement is true or false
ASE-Style Multiple Choice Fenders are bolted to all of the following EXCEPT:
A. the energy absorber B. the cowl behind the door and under the car C. the inner fender panel in the engine compartment D. the radiator core support