Equal volumes of hydrogen and helium gas are at the same pressure. The atomic mass of helium is four times that of hydrogen. If the total mass of both gases is the same, the ratio of the temperature of helium (He) to that of hydrogen (H2) is
A.
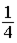 .
.B.
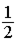 .
.C.
D.
E.
Answer: B
You might also like to view...
Which of the following order from largest to smallest is incorrect?
a. void, supercluster, star b. filament, planet, asteroid c. wall, star, planet d. void, solar system, cluster
The magnitude of the total negative charge on the electrons in 1 mol of helium (atomic number 2, atomic mass 4) is:
A) 4.8 × 104 C B) 9.6 × 104 C C) 1.9 × 105 C D) 3.8 × 105 C E) 7.7 × 105 C
The heater element of a particular 120-V toaster is a 8.9-m length of nichrome wire, whose diameter is 0.86 mm. The resistivity of nichrome at the operating temperature of the toaster is 1.3 × 10-6 ? ? m
If the toaster is operated at a voltage of 120 V, how much power does it draw? A) 720 W B) 700 W C) 750 W D) 770 W E) 800 W
Two wires A and B with circular cross sections are made of the same metal and have equal lengths, but the resistance of wire A is three times greater than that of wire B. What is the ratio of the radius of A to that of B?
1.3 2.?3 3.1 4.1/?3 5.1/3