Does this system exhibit positive or negative deviations from Raoult’s Law? Explain your answer.
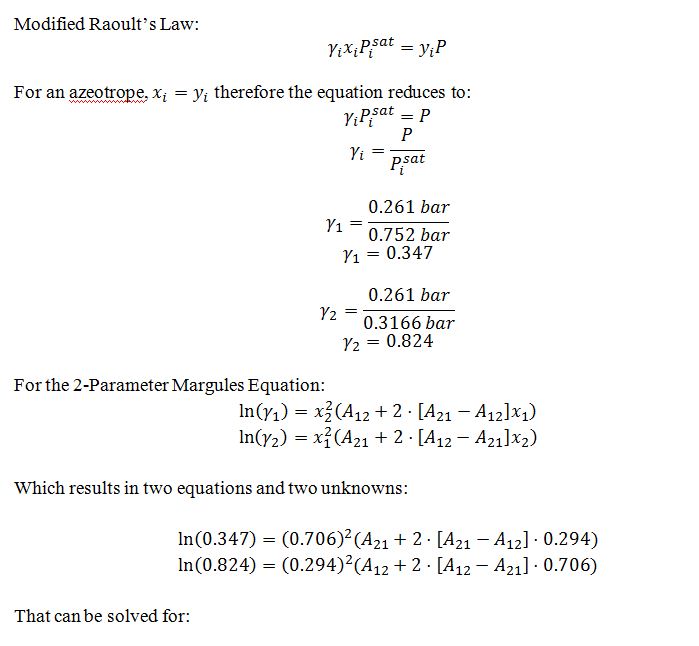
A liquid mixture of 60% by mole cyclohexanone(1) and the rest phenol (2) is in equilibrium with its vapor at 144°C. At this temperature, the vapor pressure of the cyclohexanone is 0.752 bar and that of the phenol is 0.3166 bar. Note that the system does form an azeotrope at this temperature whose composition is 29.4% by mole cyclohexanone and whose pressure is 0.261 bar. Given the above information, estimate the equilibrium pressure and vapor-phase composition of this system.


You might also like to view...
50mm to inches
What will be an ideal response?
A career as a fish and wildlife technician is for individuals who like working with animals
Indicate whether the statement is true or false
Which of these animals does NOT have a monogastric digestive system?
a. Sheep c. Cat b. Pig d. Bird
Approximately what percentage of total freight volume is handled by trucks nationwide?
A. 10% B. 50% C. 80% D. 95%