A mass of 500 lbm of 40 wt % sulfuric acid solution at 140°F is diluted with 200 lbm of pure water at 100°F.
A) What is the concentration of the resulting solution?
B) What is the heat effect (Q) of this mixing if the mixing is done such that the resulting solution is at 100°F?
C) If the mixing was done adiabatically, what would be the resulting solution temperature?
A) Find the mass of sulfuric acid in the original solution before it is diluted:
0.4(500 lbm)=200 lbm
Find the concentration of the new solution:

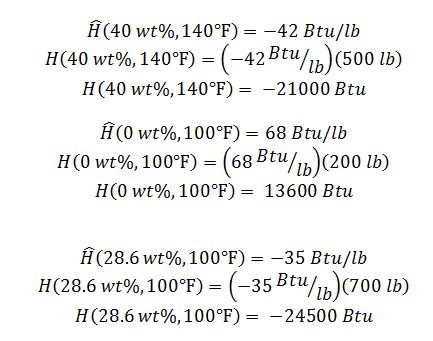
Simplified E.B.:
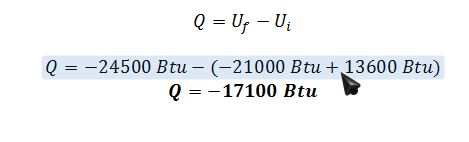
C) Simplified E.B.:
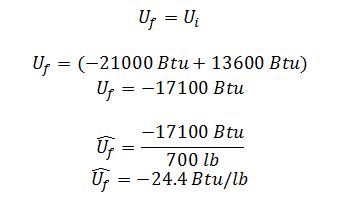
Find the temperature line that intersects with 28.6 wt% H2SO4 and -24.4 Btu/lb.
T~120?
?
You might also like to view...
IDENTIFY TEST PROCESS SYMBOLS AND DIAGRAMS

Horticulture involves the cultivation of a large number of and variety of crops
Indicate whether the statement is true or false
Pollution coming from a specific place is called _____ pollution
A) point source B) specific source C) soil source D) general source
All overhead valve engines ________
A) Have the valves located in the head B) Operate by the two-stroke cycle C) Use an overhead camshaft D) Use the camshaft to close the valves