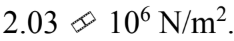The specific heat of ice is 2.100 kJ/kg  the heat of fusion for ice at 0
the heat of fusion for ice at 0 is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg
is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg  and the heat of vaporization of water at 100
and the heat of vaporization of water at 100 is 2,256 kJ/k. What is the final equilibrm temperature when 10.00 grams of ice at -15.00
is 2,256 kJ/k. What is the final equilibrm temperature when 10.00 grams of ice at -15.00 is mixed with 2.00 grams of steam at 100.0
is mixed with 2.00 grams of steam at 100.0 ?
?
A. 27.54
B. 33.79
C. 40.12
D. 45.71
E. 53.53
B. 33.79
You might also like to view...
Energy balance in the Sun refers to a balance between
A) the rate at which fusion generates energy in the Sun's core and the rate at which the Sun's surface radiates energy into space. B) the mass that the Sun loses each second and the amount of mass converted into energy each second. C) the force of gravity pulling inward and the force due to pressure pushing outward. D) the amount of energy the Sun radiates into space and the amount of energy that reaches Earth.
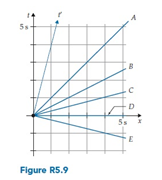
The Other Frame is moving in the +x direction with x-velocity ? = 0.25 with respect to the Home Frame. The two-observer space time diagram in figure R5.9 shows the diagram t and x axes of the Home Frame and the diagram t? axis of the Other Frame. Which of the choices in that Figure best corresponds to the diagram x? axis?
There are samples of two different isotopes, X and Y. Both contain the same number of radioactive atoms. Sample X has a half-life half that of Y. How do their decay rates compare?
a. The rates of X and Y are equal. b. X has a greater rate than Y. c. The rate depends on atomic number, not half-life. d. X has a smaller rate than Y.
A force of 100 N is applied to a rod with a diameter of 6.00 mm. The stress in the rod is
a. 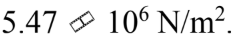
b. 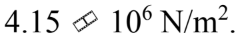
c. 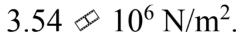
d. 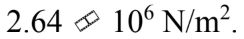
e.