Calculate the total entropy change that occurs when 2.00 kg of lead at 40.0°C are placed in a very large quantity of water at 10.0°C. The specific heat of lead is 0.031 cal/(g?K)
A)
190 J/K
B)
100 J/K
C)
6.6 J/K
D)
6.2 J/K
E)
1.4 J/K
E
You might also like to view...
MgO is a high-temperature ceramic material that has mixed ionic and covalent bonding. Should MgO have the NaCl structure or the CsCl structure based upon ionic radii?
What will be an ideal response?
A spectral line produced in the atmosphere of a star may be seen as broad whereas interstellar _______________ lines are very narrow
a. absorption b. continuous c. emission d. All of the other choices are correct.
Series/Parallel Circuits: For the circuit shown in the figure, the battery is ideal and all quantities are accurate to two significant figures. Find the current through (a) the 1.0-? resistor, (b) the 3.0-? resistor, and (c) the 4.0-? resistor?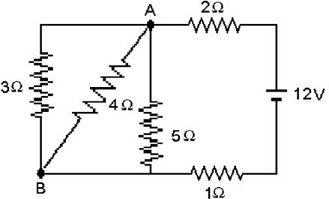
What will be an ideal response?
The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is about 29 years. Suppose you have samples of both isotopes, and that they initially contain equal numbers of atoms of these isotopes
How will the activities (number of decays per second) of the samples compare? A) The activity of the cobalt-60 sample will be greater. B) The activities cannot be compared without more information. C) The activities will be equal. D) The activity of the strontium-90 sample will be greater.