How is sublimation from a solid to a vapor different from the boiling process?
What will be an ideal response?
In principle the sublimation process is the same as the boiling process in that a
pure substance becomes a saturated vapor and possible a superheated vapor. In
boiling saturated liquid becomes saturated vapor whereas in sublimation a
saturated solid becomes a saturated vapor.
You might also like to view...
Imagine you throw a tennis ball vertically into the air. At the exact top of its trajectory it is at rest. What is the magnitude of its acceleration at this point?
A. 9.8 m/s2
B. –9.8 m/s2
C. 0 < 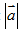 < 9.8 m/s2
< 9.8 m/s2
D. 0
E. Other (specify)
A common 5-L metal can will float in air if it is
A) evacuated of air. B) filled with a very large amount of helium. C) thrown high enough into the air. D) Nonsense! Unless the can and displaced air weigh the same, the can can't float in air.
Flatten a spherical meatball into a hamburger and you increase its
A) surface area. B) volume. C) both of these D) none of these
Exhibit 17-2
The figure below shows a sine wave on a string at one instant of time.
?
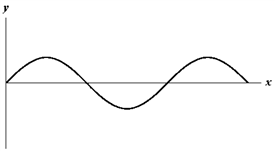
A.
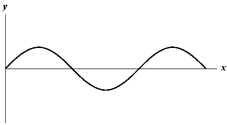
B.
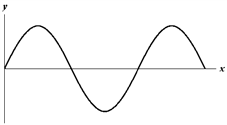
C.
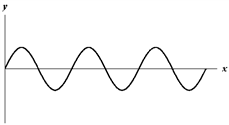
D.
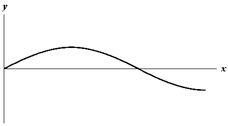
E.