Water at atmospheric pressure is boiling in a pot with a flat copper bottom on an electric range which maintains the surface temperature at 115°C. Calculate the boiling heat transfer coefficient.
GIVEN
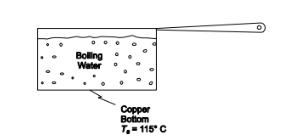
? Water at atmospheric pressure boiling in a copper bottom pot
? Surface temperature of the pot bottom (Ts ) = 115°C
FIND
? The boiling heat transfer coefficient (hb)
ASSUMPTIONS
? Temperature of the pan bottom is uniform
? The copper is polished
SKETCH
PROPERTIES AND CONSTANTS
From Appendix 2, Table 13, for saturated water at 1 atm (Tsat = 100°C)

Assuming the boiling is nucleate boiling, the heat flux, q" , is given by Equation (9.2)
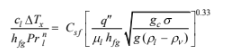
where
??Tx = Ts – Tsat = 115°C – 100°C = 15°C
gc = 1.0 (in the SI system)
n = 1.0 for water
g = 9.8 m/s2
Rearranging
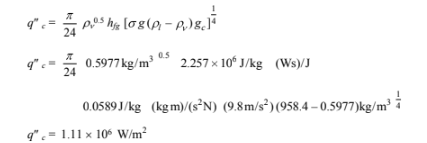
The critical heat flux for nucleate boiling is given by Equation (9.4)
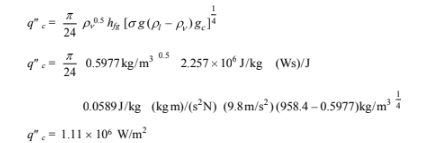
Since q"< q?c, the nucleate boiling assumption is valid.
By definition
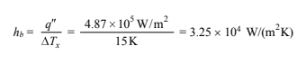
You might also like to view...
Which planet's rotation axis is closest to its orbital plane?
A. Saturn B. Venus C. Uranus D. Jupiter E. Earth
Which will NOT bend when moving in a magnetic field?
A) alpha particle B) beta particle C) gamma ray D) all bend the same
The surface of Io looks most like the pack ice of the Arctic Ocean of Earth
Indicate whether the statement is true or false
What is the formula of a compound formed from the ions M2+ and X2-?
a. M2X b. MX c. M2X2 d. MX2