Entropy: A 700-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 330 K. The compression reduces the volume of the gas from 0.40 m3 too 0.15 m3. The molecular mass of the gas is 38.0 g/mol. What is the entropy change for the gas during this compression? (R = 8.31 J/mol ? K)
A. -150 J/K
B. -57 J/K
C. 150 J/K
D. 57 J/K
E. 0 J/K
Answer: A
You might also like to view...
Sunspots are known to be magnetic phenomena because
a. Doppler shifts in spectral lines are observed. b. the Zeeman effect is observed in sunspots. c. collisional broadening is observed in spectral lines. d. infrared observations indicate that the sunspots are cooler than their surroundings. e. observations during eclipses reveal a very extensive photosphere.
Size of the Nucleus: Calculate the estimated density of the nucleus of 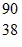 Sr? (mneutron ? mproton = 1.67 × 10-27 kg)
Sr? (mneutron ? mproton = 1.67 × 10-27 kg)
A. greater than 3.0 × 1020 kg/m3 B. 90 kg/m3 C. 2.1 × 1019 kg/m3 D. 2.0 × 1015 kg/m3 E. 2.3 × 1017 kg/m3
Heat Engines: During each cycle, a heat engine takes in 4.0 J of heat, does 1.0 J of work, and expels 3.0 J of heat. What is its efficiency?
Fill in the blank(s) with the appropriate word(s).
You have taken a picture of a galaxy 1 billion light years away. That picture shows
A) a galaxy that is 1 billion years old. B) a galaxy that is 1 billion light years old. C) a galaxy that is 13 billion years old. D) a galaxy as it was 1 billion years ago.