Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution?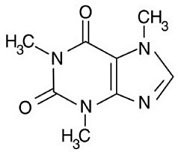
A. A second layer of water would form.
B. Nothing, and the HCl gas would merely bubble out of solution.
C. The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.
D. The diethyl ether insoluble caffeine salt would form as a white precipitate.
Answer: D
You might also like to view...
In the above map, the darker areas most likely represent areas of higher
A) birth rates. B) death rates. C) refugee populations. D) education levels. E) nutritional densities.
What is the linear distance from the 1400-ft elevation contour in Trail Canyon to the 200-ft contour? What is the relief between these two points?
What will be an ideal response?
Which type of ionizing radiation is generally the most dangerous?
A) Alpha radiation B) Omega radiation C) Gamma radiation D) Beta radiation
In terms of pedogenic regimes, the leaching process in humid and warm climates is known as
A) laterization B) gleization. C) salinization. D) calcification. E) podzolization.