For one complete cycle of a reversible heat engine, which of the following quantities is NOT zero?
A. the change in the entropy of the working gas
B. the change in the pressure of the working gas
C. the change in the internal energy of the working gas
D. the work done by the working gas
E. the change in the temperature of the working gas
Answer: D. the work done by the working gas
You might also like to view...
A(n) _______________ can thrive in extreme conditions, such as ice-covered lakes in Antarctica, far underground inside solid rock, amongst the cinders of summits of extinct volcanoes, or in pools of acids
Fill in the blank(s) with correct word
Why do you think that more plants per capita in Europe than in the United States burn municipal solid waste? Give your reasons, and explain the differences
What will be an ideal response?
Larger batteries have higher output voltages
Indicate whether the statement is true or false
What is the direction of 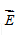 at point Q?
at point Q?
A. A B. B C. C D. D E. E F. F T. Zero