Solve the problem.In thermodynamics, the differential form of the internal energy of a system is dU = T dS - P dV, where U is the internal energy, T is the temperature, S is the entropy, P is the pressure, and V is the volume of the system. The First Law of Thermodynamics asserts that dU is an exact differential. Using this information, justify the thermodynamic relation 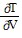 = -
= - 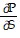 .
.
What will be an ideal response?
Answers will vary.
You might also like to view...
Refer to this figure to decide whether the statement is true or false. 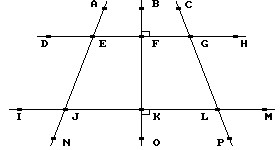
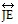 is parallel to
is parallel to 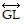 .
.
A. True B. False
Evaluate the expression for the given values. Expression your answer as a fraction unless otherwise indicated.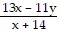 x = 6, y = 4
x = 6, y = 4
A. 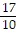
B. 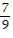
C. 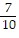
D. 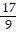
Find an equation for the line, in the indicated form, with the given properties. Containing the points (6, -8) and (0, 3); general form
A. -11x + 6y = 18 B. -14x + 3y = -9 C. 14x - 3y = -9 D. 11x + 6y = 18
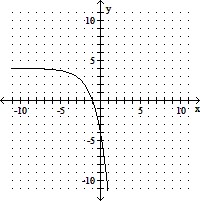
Use transformations to graph the function. Determine the domain, range, and horizontal asymptote of the function.f(x) = -2x+3 + 4
A. domain of f: (-?, ?); range of f: (-4, ?);
horizontal asymptote: y = 4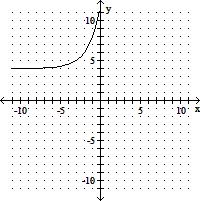
B. domain of f: (-?, ?); range of f: (-?, -4);
horizontal asymptote: y = -4 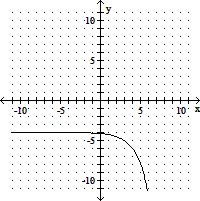
C. domain of f: (-?, ?); range of f: (-?, -4);
horizontal asymptote: y = -4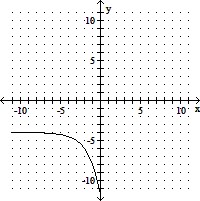
D. domain of f: (-?, ?); range of f: (-?, 4);
horizontal asymptote: y = 4