The Beer-Lambert law relates the absorbance A of a solution to the concentration C of a species in solution by A = MLC, where L is the path length and M is the molar absorption coefficient. Assume that C = 1.25 ± 0.03 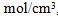 L = 1.2 ± 0.1 cm, and A = 1.30 ±0.05.
L = 1.2 ± 0.1 cm, and A = 1.30 ±0.05.
a. Estimate M and find the uncertainty in the estimate.
b. Which would provide a greater reduction in the uncertainty in M: reducing the uncertainty in C to 0.01 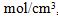 reducing the uncertainty in L to 0.05 cm, or reducing the uncertainty in A to 0.01?
reducing the uncertainty in L to 0.05 cm, or reducing the uncertainty in A to 0.01?
You might also like to view...
Technician A says that E3 actuators initially draw around 10 amps when first switched. in the hold status position. Technician B says that E3 actuators draw around 4 amps to hold position. Who is correct?
A. Technician A only B. Technician B only C. Both A and B D. Neither A nor B
What are the two types of landscape fences and how are they used?
What will be an ideal response?
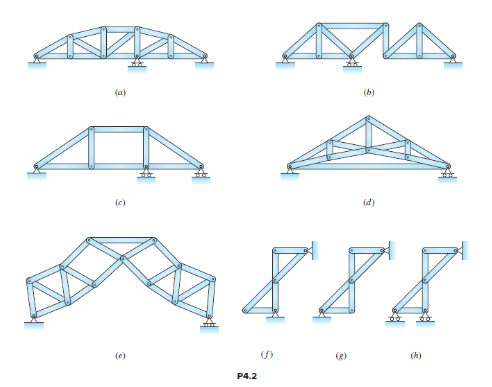
Classify the trusses in Figure P4.2 as stable or unstable. If stable, indicate if determinate or indeterminate. If indeterminate, indicate the degree.
Plant symptoms such as aborted fruits, dead terminal buds, and misshapen new leaves suggest a deficiency of which micronutrient?
A) Cu B) Mn C) Fe D) B E) Zn