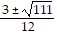The pH of a solution is given by the formula 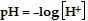 , where
, where  is the concentration of hydrogen ions in moles per liter in the solution. Use this formula to solve the problem.Lower levels of pH indicate a more acidic solution. If the pH of solution A is 4.2 and the pH of solution B is 6.9, how much more acidic is solution A than solution B?
is the concentration of hydrogen ions in moles per liter in the solution. Use this formula to solve the problem.Lower levels of pH indicate a more acidic solution. If the pH of solution A is 4.2 and the pH of solution B is 6.9, how much more acidic is solution A than solution B?
A. 0.0000630 times as acidic
B. 501.2 times as acidic
C. 0.00200 times as acidic
D. -0.0000630 times as acidic
Answer: B
You might also like to view...
When a car skids to a stop, the length L, in feet, of the skid marks is related to the speed S, in miles per hour, of the car by the equation
?
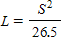
What will be an ideal response?
Determine the natural logarithm of the number. Round to the nearest ten-thousandth.244
A. 90.0369 B. 5.4972 C. 2.3874 D. 0.1814
Solve.4x - 3 = 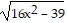
A. -2 B. 4 C. 2 D. empty set solution
Use the quadratic formula to solve the quadratic equation.6x2 + 3x + 5 = 0
A. x = 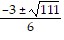
B. x = 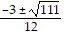
C. no real solution
D. x =