Show that for an ideal gas mixture maintained at a constant temperature and pressure, the molar concentration C of the mixture remains constant, but this is not necessarily the case for the density ? of the mixture.
What will be an ideal response?
The ideal gas relation can be expressed as 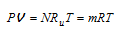 where Ru is the universal gas constant, whose value is the same for all gases, and R is the gas constant whose value is different for different gases. The molar and mass densities of an ideal gas mixture can be expressed as
where Ru is the universal gas constant, whose value is the same for all gases, and R is the gas constant whose value is different for different gases. The molar and mass densities of an ideal gas mixture can be expressed as
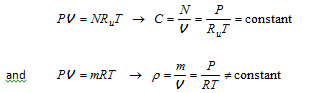
Therefore, for an ideal gas mixture maintained at a constant temperature and pressure, the molar concentration C of the mixture remains constant but this is not necessarily the case for the density ? of mixture.
You might also like to view...
Answer the following statement(s) true (T) or false (F)
1. The number of accelerating contactors depends on the desired number of speeds for the motor. 2. The motor starts slowly with full resistance in the primary circuit. 3. The speed may be changed from medium to low or high to low by pressing the medium-speed push button. 4. The resistors should have a high enough power rating to operate at any speed.
The most common type of film plastic used in the United States is _______________ The thickness of this plastic for the outer layer should be _____________ and for the inner layer it only needs to have a thickness of ___________. This plastic is mainly destroyed over time by _______ . Its life expectancy can be up to _______ years
Fill in the blank(s) with correct word
List three types of power sources used to operate power tools.____________________________________________________________________________________________________________
What will be an ideal response?
Chlorofluorocarbons are being discussed. Technician A says CFC's production and use is strictly regulated by the Clean Air Act. Technician B says chlorofluorocarbons are an artificially made chemical. Who is correct?
A. Technician A only B. Technician B only C. Both A and B D. Neither A nor B