One cubic meter of argon is taken from 1 bar and 25°C to 10 bar and 300°C by each of the following two-step paths. For each path, compute Q, W, ?U, and ?H for each step and for the overall process. Assume mechanical reversibility and treat argon as an ideal gas with 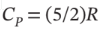 and
and 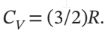
What will be an ideal response?
(a) Isothermal compression followed by isobaric heating.
(b) Adiabatic compression followed by isobaric heating or cooling.
(c) Adiabatic compression followed by isochoric heating or cooling.
(d) Adiabatic compression followed by isothermal compression or expansion.
a. Argon goes from 1 bar and 25 C isothermally compressed to 10 bar, and isobaric heating to 300 C. First, the volume at the initial state ( 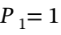 bar and
bar and 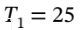 25 C) is the
25 C) is the 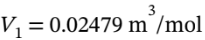 (from V = RT/P). The volume at the final state
(from V = RT/P). The volume at the final state 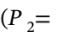 10 bar and
10 bar and 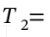 300 C)is the
300 C)is the 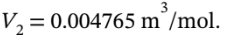
So for the isothermal compression, ?U = 0, Q = 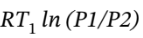 = -5707 J/mol,
= -5707 J/mol,
W = -Q = -(-5707 J/mol) = 5707 J/mol, and ?H =0.
For the isobaric heating, ?U = Cv?T = 1.5* R * (573.15 K – 298.15 K) = 3429 J/mol,
?H = Q = Cp?T = 2.5* R * (573.15 K – 298.15 K) = 5715 J/mol,
W = -R?T = R * (573.15 K – 298.15 K) = -2286 J/mol.
Altogether, W =5707 J/mol – 2286 J/mol = 3421 J/mol, ?U =3429 J/mol, Q = -5707 J/mol + 5715 J/mol = 8 J/mol, ?H = 5715 J/mol.
b. Argon goes from 1 bar and 25 C adiabatically compressed to 10 bar, and isobaric heating to 300 C. Use the same volumes as in part (a).
So for the adiabatic compression, Q = 0,
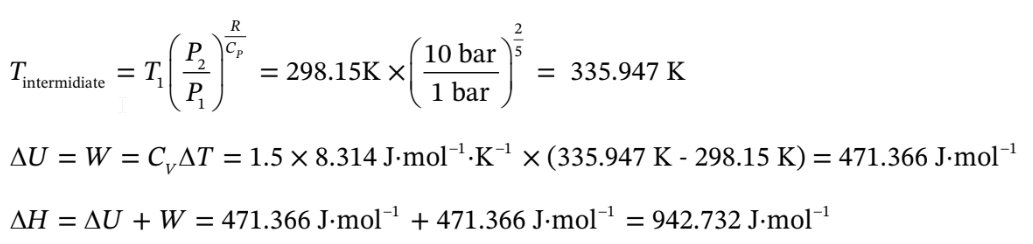
For the isobaric heating,
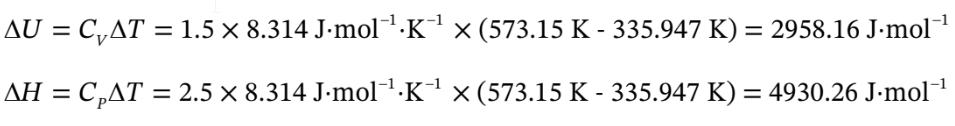
?H = Q = 4930.26 J/mol,
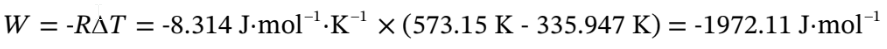
Altogether,
W =-1972.11 J/mol +471.366 J/mol = -1500.74 J/mol,
?U =471.366 J/mol + 2958.16 J/mol = 3429.52 J/mol,
Q = 4930.26 J/mol,
?H = 4930.26 J/mol + 942.732 J/mol = 5873 J/mol.
c. Argon goes from 1 bar and 25 C adiabatically compressed to 10 bar, and isochoric heating to 300 C. Use the same volumes as in part (a).
So for the adiabatic compression, Q = 0,

For the isochoric heating,
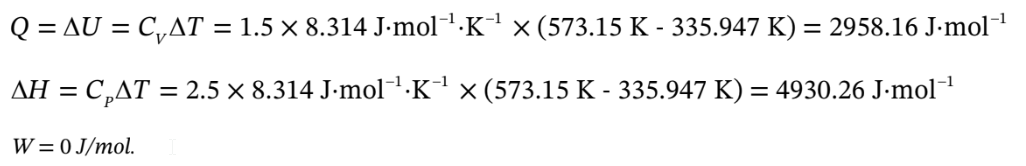
Altogether, W =471.366 J/mol,
?U =471.366 J/mol + 2958.16 J/mol = 3429.52 J/mol,
?H = 4930.26 J/mol + 942.732 J/mol = 5873 J/mol.
Q = 2958.16 J/mol.
d. Argon goes from 1 bar and 25 C adiabatically compressed to 300C, and isothermal compression to 10 bar.
Use the same volumes as in part (a).
So for the adiabatic compression, Q = 0,
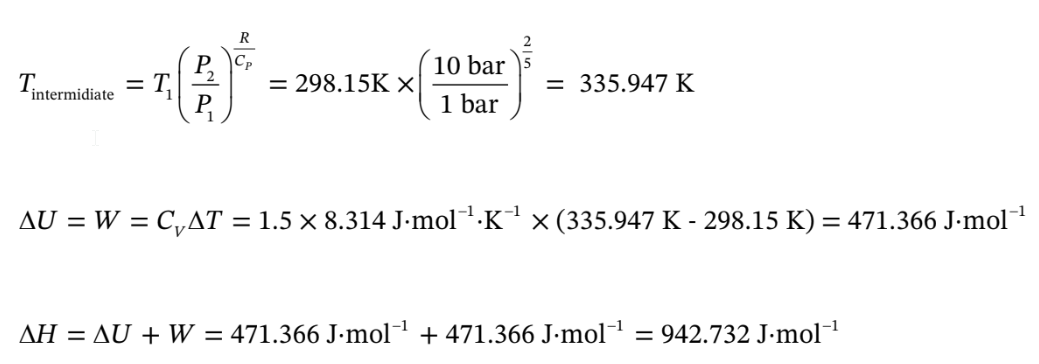
For the isothermal compression, ?H = ?U = 0,
Q = 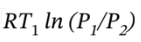 = -5707 J/mol, and W = -Q = 5707 J/mol
= -5707 J/mol, and W = -Q = 5707 J/mol
Altogether, W =471.366 J/mol + 5707 J/mmol = 6179.056 J/mol, ?U =471.366 J/mol, Q = -5707J/mol, ?H = 942.732 J/mol.
You might also like to view...
Getting the parts of an assembly properly positioned may take more time than it takes to do the welding.
Answer the following statement true (T) or false (F)
Conduit is best stored _____.
a. in loose piles sorted by size b. unsealed to prevent bulging due to temperature changes c. in pipe racks d. outdoors to become acclimated to the ambient temperature
A demultiplexer accepts data from:
A) multiple input lines and transfers it to one output line. B) one input line and transfers it to one output line after conditioning and buffering the data signals. C) one input line and transfers it to multiple output lines. D) sequential input lines and transfers it to one output line.
Which of the following is not one of the five steps included in the REGREEN program?
A) Project completion audit B) Building assessment C) Client interview D) Cost-benefit analysis