Which of the following statements is true?
A. The change in internal energy of an ideal gas can be calculated using the equation dU = CPdT, even if the pressure of the gas isn’t constant.
B. The change in enthalpy of a supercritical fluid can be calculated using the equation dH = CPdT, even if the pressure of the fluid isn’t constant.
C. The expression CP = CV + R was derived for ideal gases, but is frequently applied to liquids and solids as well.
D. The properties tabulated in the steam tables are all state properties, but none of them are extensive properties.
E. WEC = -PV, so U - WEC is equal to H.
A. Incorrect. The equation is not correct as written; review Equation 2.32 and Section 2.3.2.
B. Incorrect. Supercritical fluids are by definition at high pressures. One cannot reasonably apply an ideal gas model to them.
C. Incorrect. NEVER use this equation for anything other than ideal gases. The assumptions made in deriving (Reread Section 2.3.3, especially Example 2-4) make it impossible to be accurate for other states of matter.
D. Correct. Extensive properties are dependent on the mass of the system, but the data in the steam tables is expressed as intensive properties (kJ/kg for example).
E. Incorrect. WEC is equal to the integral of –PdV. This only equals –PV if P is constant with respect to volume.
You might also like to view...
The bearing capacity of a soil is generally expressed in terms of
A) pounds or kips. B) pounds or kips per square foot. C) pounds or kips per inch. D) pounds or kips per square inch. E) pounds or kips per cubic inch.
Discuss the advantages and disadvantages of hunting
What will be an ideal response?
Basic floral design shapes include Hogarth's (S) curve and right angle
Indicate whether the statement is true or false
Label J represents
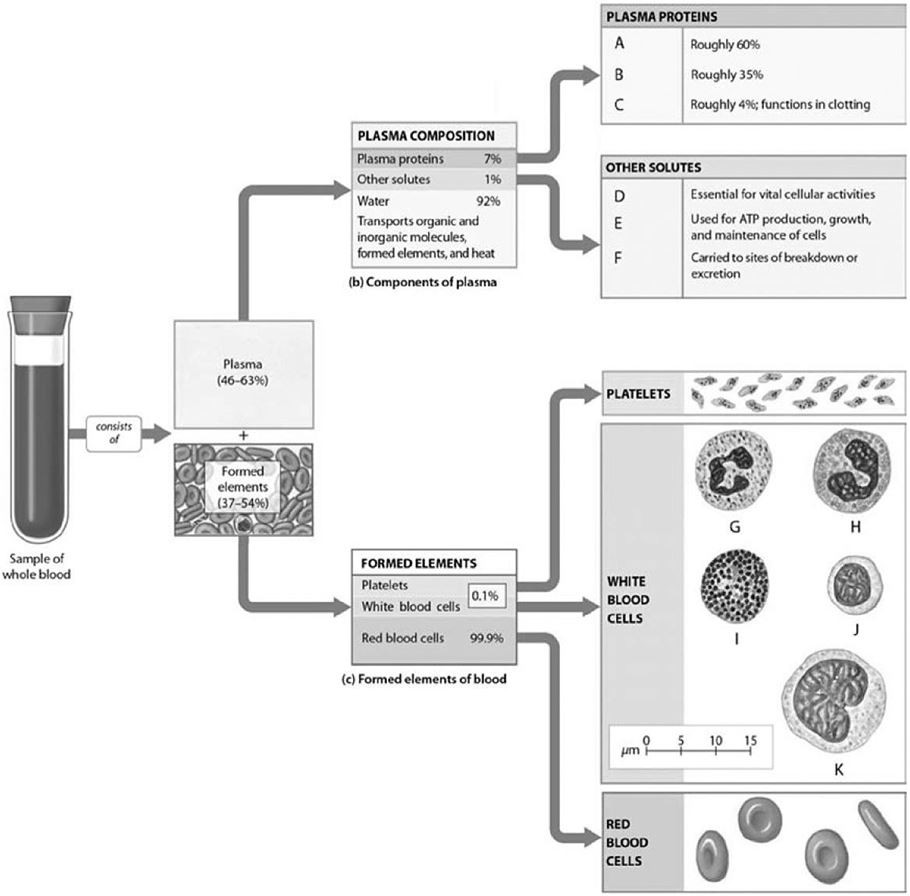
A) basophils.
B) neutrophils.
C) monocytes.
D) eosinophils.
E) lymphocytes.