Of the following statements about water's latent heat and changes in state, which is/are true?
-When water vapor condenses and forms a liquid, its latent heat of condensation releases heat to the environment; this is what powers hurricanes.
-In the vapor state, there are no weak (hydrogen) bonds between water molecules.
-In the solid state, all water molecules are connected by weak (hydrogen) bonds.
-In all three states of matter, there are no strong (covalent) bonds.
-When water evaporates, its latent heat of evaporation absorbs heat.
-Latent heat allows water to transfer energy from one place to another.
-When water vapor condenses and forms a liquid, its latent heat of condensation releases heat to the environment; this is what powers hurricanes.
-In the vapor state, there are no weak (hydrogen) bonds between water molecules.
-In the solid state, all water molecules are connected by weak (hydrogen) bonds.
-When water evaporates, its latent heat of evaporation absorbs heat.
-Latent heat allows water to transfer energy from one place to another.
You might also like to view...
Nitrites used in food processing and wine production can act as a mutagen and cause ____________________
Fill in the blanks with correct word
In general, the environmental lapse rate:
a. is a constant. b. can vary because of atmospheric conditions. c. is of little importance to our weather. d. none of these
________ accounts for greater ocean depths moving away from the oceanic ridge toward the deep ocean basin
A) Mantle plume B) Convection C) Thermal contraction D) Gravity
The Csa climate of Athens Greece owes its summer precipitation pattern to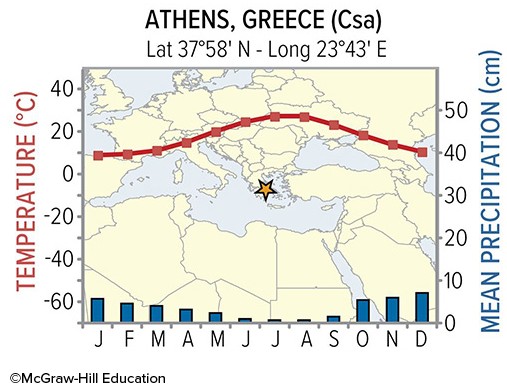
A. seasonal shifts in wind direction. B. hurricanes that bring high amounts of rain. C. the moist western side of the subtropical high. D. the dry eastern side of the subtropical high.