Why does iodine, I2(s), spontaneously sublime at room temperature?
A) The contact of iodine, I2(s), with the water vapor in the air causes the sublimation process.
B) Iodine, I2(s), pulls Image from the air as it self-oxidizes under sublimation to I2(g).
C) There is an increase in entropy as solid iodine, I2(s), sublimes into iodine vapor, I2(g), because in the gaseous phase energy is more readily dispersed.
D) The reaction of I2(s) subliming to I2(g) is highly exothermic and therefore proceeds spontaneously.
Answer: C
You might also like to view...
How much energy does a 100-W light bulb use in 8.0 hours?
A) 13 kWh B) 0.80 kWh C) 0.0080 kWh D) 800 kWh
Coulomb's Law: An asteroid of mass 58,000 kg carrying a negative charge of 15 ?C is 180 m from a second asteroid of mass 52,000 kg carrying a negative charge of 11 ?C. What is the magnitude of the net force the asteroids exert upon each other, assuming we can treat them as point particles? (G = 6.67 × 10-11 N ? m2/kg2, k = 1/4 ??0 = 8.99 × 109 N ? m2/C2)
A. 0.000040 N B. 0.0062 N C. 570,000 N D. 510,000 N
The equivalent capacitance of the circuit shown below is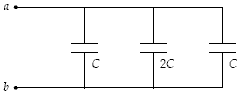
A. 0.2 C. B. 0.4 C. C. 1 C. D. 4 C. E. 5 C.
Force on a Moving Charge: A proton is to orbit Earth at the equator using Earth's magnetic field to supply part of the necessary centripetal force. In what direction should the proton move?
A. upward B. northward C. southward D. eastward E. westward