Assuming ideal gas behavior, determine the mass of each gas listed below, if the gas occupies 0.5 ft3, at 100 psi and 540 R:
(a) hydrogen,
(b) methane,
(c) nitrogen,
(d) argon
(e) carbon dioxide
Given: V = 0.5 ft3; P = 100 psi; T = 540 R
What will be an ideal response?
From the ideal gas law, m = RT/PV
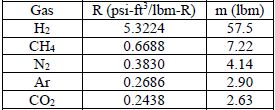
The table below gives the results for each gas
You might also like to view...
Which of the following is most likely to be a health risk as a result of obesity?
a. high blood pressure c. osteoporosis b. type 1 diabetes d. allergies
Managing wildlife involves making decisions about what three key questions?
What will be an ideal response?

A. X B. Y C. Z D. None of the above
_________________________ are case-hardened cap screws that secure the seat track to the floor structure.
Fill in the blank(s) with the appropriate word(s).