If two gases have molar masses 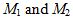 , Graham’s law states that the ratio R of their rates of effusion through a small opening is given by
, Graham’s law states that the ratio R of their rates of effusion through a small opening is given by 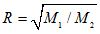 . The effusion rate of an unknown gas through a small opening is measured to be 1.66 ± 0.03 times greater than the effusion rate of carbon dioxide. The molar mass of carbon dioxide may be taken to be 44 g/mol with negligible uncertainty.
. The effusion rate of an unknown gas through a small opening is measured to be 1.66 ± 0.03 times greater than the effusion rate of carbon dioxide. The molar mass of carbon dioxide may be taken to be 44 g/mol with negligible uncertainty.
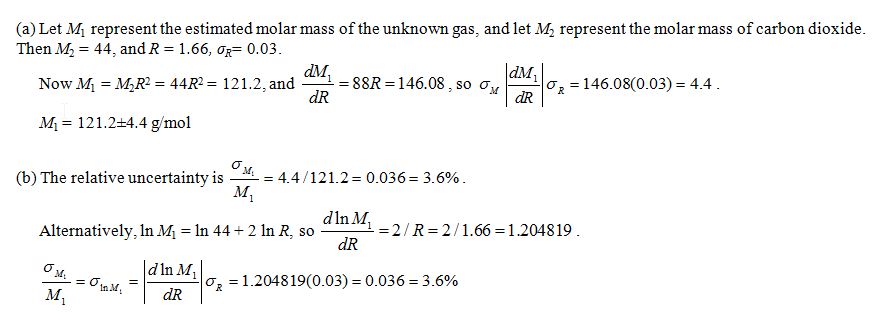
a. Estimate the molar mass of the unknown gas, and find the uncertainty in the estimate.
b. Find the relative uncertainty in the estimated molar mass.
Trades & Technology
You might also like to view...
The air we breathe is mostly oxygen.
Answer the following statement true (T) or false (F)
Trades & Technology
Among the places Alexander conquered, besides Persia, were
a. Egypt, Spain, and Carthage. b. Phoenicia, Gaza, and Egypt. c. Gaza, Italy, and Phoenicia. d. Tyre, Gaza, and Dacia.
Trades & Technology
Powder metallurgy process is less cost effective than casting, forging and stamping.
Answer the following statement true (T) or false (F)
Trades & Technology
UV lights can cause severe burns.
Answer the following statement true (T) or false (F)
Trades & Technology