Which of the following statements is accurate?
An ammonia synthesis process works as follows: Fresh feed air and hydrogen are combined with a recycle stream and the combined stream is compressed to 10 MPa. The compressed stream enters a reactor, and a fraction of the entering nitrogen and hydrogen are converted into ammonia. The reactor effluent, still at 10 MPa, enters a condenser. The liquid and vapor streams leaving the condenser are in VLE. The liquid stream, which is almost pure ammonia, is the product. A fraction of the vapor stream leaving the condenser is purged, while the rest is recycled.
A. Raoult’s Law would be a reasonable way to model the VLE for ammonia in the condenser.
B. Henry’s Law would be a reasonable way to model the VLE for oxygen in the condenser.
C. If we increase the size of the reactor, allowing a larger fraction of the nitrogen and hydrogen to be converted to ammonia, it won’t lead to an increase in the liquid product flow rate, because the product flow rate is limited by the equilibrium constraint in the condenser.
D. If we maintain the same flow rate of entering feeds, but we purge a smaller fraction of the vapor leaving the condenser, then the mole fraction of oxygen in the purge stream will go down.
E. None of the above are true.
A. Incorrect. Raoult’s Law incorporates the ideal gas law for vapor phase modeling. A pressure of 10 MPa is far too high to be considered ideal.
B. Correct. The liquid stream is described as almost pure ammonia. Henry’s Law is used to model a system in which a trace of gas dissolved in a liquid. It might well be acceptable to assume the liquid is pure ammonia, but if one was going to account for the presence of gases dissolved in the ammonia, Henry’s Law would indeed be a reasonable way to model the VLE.
C. Incorrect. The “equilibrium constraint” in VLE involves mole fractions, not absolute numbers of moles. If the flow rate of ammonia entering the condenser increases, then the liquid product flow rate can increase without a change in the mole fraction of ammonia in either the liquid or the vapor product.
D. Incorrect. The amount of oxygen purged (mol/time) essentially equals the amount of oxygen entering at steady state, since none is consumed in the reaction and very little leaves through the liquid product. If we lower the total flow rate of the purge stream, then the mole fraction of oxygen in that stream, which is constant, will increase.
E. Incorrect. One of these statements is reasonable.
You might also like to view...
Vehicle heater hoses are attached to the ________.
A) Heater core B) Engine C) Either A or B but not both D) Both A and B
The Pierce administration's secret scheme to gain control of Cuba was stopped when
A. Spain threatened a preemptive war against the United States. B. the secret Ostend Manifesto was leaked to the public. C. United States leaders signed the Clayton-Bulwer Treaty. D. Spain declared that it would abolish slavery in Cuba. E. United States adventurers bungled their invasion.
An RLC series circuit has an applied voltage of 240 volts. R = 48 ?, XL = 100 ?, XC = 36 ?, and Z = 80 ?. What is the voltage drop across the inductor?
A. 140 V B. 208 V C. 300 V D. 344 V
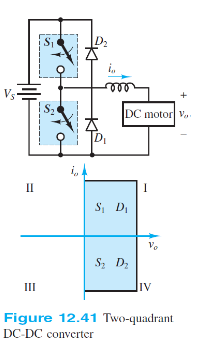
For the two-quadrant converter of Figure 12.41 assume that thyristors S1 and S2 are on for t1 and off for T t1, where T is the switching period. Derive an expression for the average output voltage voavg in terms of the supply voltage VS and the duty cycle ?.