An ideal gas initially at 300 K and 1 bar undergoes a three-step mechanically reversible cycle in a closed system. Pressure increases isothermally to 5 bar; Pressure increases at constant volume; and, the gas returns adiabatically to its initial state. Take 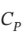 = (7/2)R and
= (7/2)R and 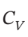 = (5/2)R.
= (5/2)R.
(a) Sketch the cycle on a PV diagram.
(b) Determine (where unknown) V, T, and P for states 1, 2, and 3.
(c) Calculate Q, W, ?U, and ?H for each step of the cycle.
a. 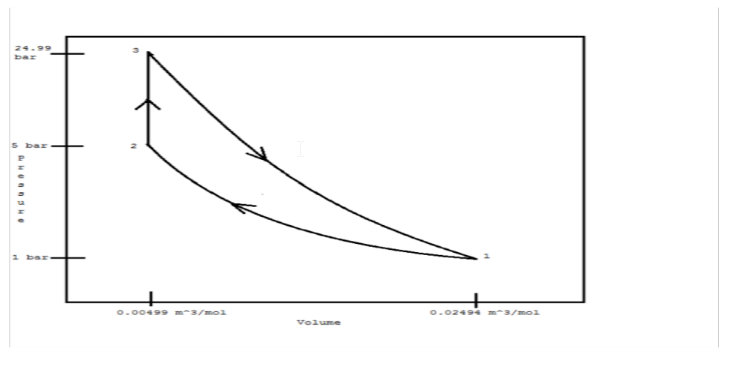
You might also like to view...
Fish is high in protein and low in calories
Indicate whether the statement is true or false.
Determine the stress on a grade 2, 5/8-11 fastener that is loaded in tension to 15,000 pounds force (lbf). Determine whether this load will create a permanent elongation or return to its original length when the load is removed.
What will be an ideal response?
All batteries should be in a secure bracket that is bolted to the vehicle to prevent physical damage to the battery
Indicate whether the statement is true or false
The control technique used in a system that makes point level measurements is the __________ method.
A. on/off B. proportional