Carbon-14 Dating: An old bone sample contains 321 g of carbon and has an activity of 947 decays/min due to the carbon-14 in it. The half-life of carbon-14 is 5730 y. Assume that the activity of the atmospheric carbon is, and has remained, 0.255 Bq per gram of carbon.(a) How old is the sample?(b) What will be the activity of the sample 2000 y from now, in decays per minute?
What will be an ideal response?
(a) 13,600 y (b) 743 decays/min
You might also like to view...
Energy in Nuclear Reactions: Consider the nuclear reaction 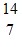 N +
N + 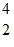 He ?
He ?  O +
O + 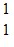 H. The known atomic masses are
H. The known atomic masses are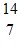 N: 14.003074 u
N: 14.003074 u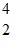
/>He: 4.002603 u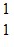 H: 1.007825 u
H: 1.007825 u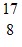 O: 16.999131 uWhat is the Q-value (or reaction energy) of this reaction? (1 u = 931.5 MeV/c2)
O: 16.999131 uWhat is the Q-value (or reaction energy) of this reaction? (1 u = 931.5 MeV/c2)
A. -1.191 MeV
B. -2.020 MeV
C. -6.725 MeV
D. -9.055 MeV
Length Contraction: A starship is constructed to be 100 m long in the factory. It is launched and as it coasts by Earth, it is measured to be shortened to 5.48 m long.(a) How fast, in terms of the speed of light, is it moving past Earth?(b) What length does the ship's crew measure for the ship?
What will be an ideal response?
Which of the following is true of planet Earth?
A) It has an atmosphere rich in water vapor. B) It has an atmosphere that is mainly composed of oxygen. C) It is the fourth largest planet of the Solar System. D) It is half the size of Jupiter.
Discuss the observational differences between spiral, elliptical, and irregular galaxies
What will be an ideal response?