The Lyman series lines of hydrogen all lie in the infrared
a. True
b. False
Indicate whether the statement is true or false
False
You might also like to view...
Projectile Motion: In an air-free chamber, a pebble is thrown horizontally, and at the same instant a second pebble is dropped from the same height. Compare the times of fall of the two pebbles.
A. The thrown pebble hits first. B. The dropped pebble hits first. C. They hit at the same time. D. We cannot tell without knowing which pebble is heavier.
Nuclear Binding Energy: One of the fusion reactions that occurs in the sun is: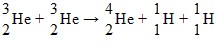 The following atomic masses are known:
The following atomic masses are known: What is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
What is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
A. 11 MeV B. 13 MeV C. 15 MeV D. 17 MeV E. 19 MeV
An automotive catalytic convertor is a packed bed in which a platinum catalyst is coated on the surface of small alumina spheres. A metal container holds the catalyst pellets and allows engine exhaust gases to flow through the bed of pellets. The catalyst must be heated by the exhaust gases to 300°C before the catalyst helps combust unburned hydrocarbons in the gases. The time required to achieve this temperature is critical because unburned hydrocarbons emitted by the vehicle during a cold start comprise a large fraction of the total emissions from the vehicle during an emission test. A fixed volume of catalyst is required but the shape of the bed can be modified to increase the heat-up rate. Compare the heat-up time for a bed 5-cm-diameter and 20-cm-long with one 10 cm diameter and
5-cm-long. The catalyst pellets are spherical, 5-mm-diameter, have a density of 2 g/cm3, thermal conductivity of 12 W/(m K) and specific heat of 1100 J/(kg K). The packed-bed void fraction is 0.5. Exhaust gas from the engine is at a temperature of 400°C, a flow rate of 6.4 gm/s, and has the properties of air.
GIVEN
FIND
The heat-up time (t) for the pellet surface temperature (Tp) to reach 300°C
ASSUMPTIONS
The initial temperature of the bed (To) = 20°C
SKETCH
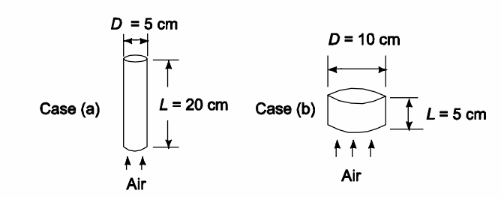
A researcher is using x-rays to investigating a cubic crystal. He is looking at Bragg reflection from the planes parallel to the cube faces
He finds that when using x-rays with a wavelength of 0.165 nm, a strong first maximum occurs when the beam makes an angle of 23.5° with the planes. What is the spacing of adjacent atoms in this crystal?