Liquids that boil at relatively low temperatures are often stored as liquids under their vapor pressures, which at ambient temperature can be quite large. Thus, n-butane stored as a liquid/vapor system is at a pressure of 2.581 bar for a temperature of 300 K. Large-scale storage 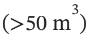 of this kind is sometimes done in spherical tanks. Suggest two reasons why.
of this kind is sometimes done in spherical tanks. Suggest two reasons why.
What will be an ideal response?
The advantages of spherical tanks stem from the fact that a spherical container has the minimum surface area for a given interior volume. Because of this:
(a) A minimum quantity of metal is required for tank construction (for a given metal thickness).
(b) The tensile stress within the tank wall is everywhere uniform, with no sites of stress concentration, and the stress is purely tensile (everywhere tangential to the tank surface). Moreover, the maximum stress within the tank wall is kept to a minimum.
(c) The surface area for heat transfer is minimized, optimizing the efficiency of cooling or insulating the tank.
You might also like to view...
Various types of algae can be processed into:
A) Diesel fuel B) Gasoline C) Ethanol D) Methanol
Define the following terms:
What will be an ideal response?
In a typical multi-orifii, hydraulic injector, what force holds the nozzle valve on its seat?
A. spring force B. fuel pressure C. combustion pressures D. compression pressures
What are some present-day applications of Wheatstone bridge circuits?
What will be an ideal response?