One mole of an ideal gas with a volume of 4.00 liters and a temperature of 0 is mixed with one mole of an ideal gas with a volume of 2.00 liters and a temperature of 100
is mixed with one mole of an ideal gas with a volume of 2.00 liters and a temperature of 100 The volume of the mixture is 6 liters. What is the N/V of the mixture?
The volume of the mixture is 6 liters. What is the N/V of the mixture?
a. 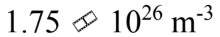
b. 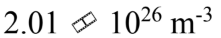
c. 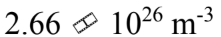
d. 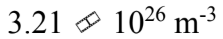
e. 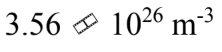
b. 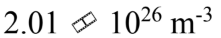
Physics & Space Science
You might also like to view...
The {100} planes of a cubic unit cell form the ______________of the cube.
Fill in the blank(s) with the appropriate word(s).
Physics & Space Science
A zero decibel sound level has zero amplitude
a. True b. False Indicate whether the statement is true or false
Physics & Space Science
Uranus rotates _______________ degrees from the _______________ to its orbit
Fill in the blank(s) with correct word
Physics & Space Science
n-Butane and isobutane are the two ______________ of C4H10
Fill in the blank(s) with correct word
Physics & Space Science