Assume that an interionic pair potential between K+ and Cl– ions can be approximated by Equation 2.15. Assume that the repulsive ion core interactions can be modeled with a power of m=12. Experimentally it has been determined that the energy to separate one pair of K+ and Cl– ions is 5.0 eV, and that the equilibrium interionic separation is equal to 0.266 nm. Use this experimental data to determine the value of B for an ion pair. Use the SI system of units for this problem.
What will be an ideal response?
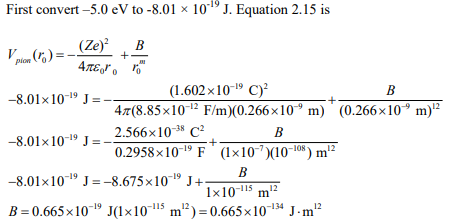
You might also like to view...
Should we expect to find abundant amounts of gold and other rare-earth elements elsewhere in the universe?
What will be an ideal response?
We are measuring the spectra of two hydrogen gas clouds. The laboratory frame wavelength of one hydrogen line is 656.2 nm. Cloud A's emission line wavelength is 660.1 nm and Cloud B's emission line wavelength is 670.1 nm
What can we conclude about these clouds? A) They are both approaching us, and Cloud A is approaching faster than Cloud B. B) They are both receding from us, and Cloud B is receding faster than Cloud A. C) They are both approaching us, and Cloud B is approaching faster than Cloud A. D) They are both receding from us, and Cloud A is receding faster than Cloud B.
When considering light as made up of individual "pieces," each characterized by a particular amount of energy, the pieces are called
A) photons. B) wavicles. C) gamma rays. D) frequencies.
An ideal gas is confined to a container with adjustable volume. The pressure and mole number are constant. By what factor will volume change if absolute temperature triples?
a. 1/9 b. 1/3 c. 3 d. 9