In a certain chemical reaction, 8.1 × 10-19 J of energy is released per molecule formed
What is the difference in mass per molecule between the sum of the masses of the molecules of the reagents before the reaction and the mass of the molecules produced by the reaction? (c = 3.0 × 108 m/s)
A) 2.7 × 10-27 kg
B) 8.1 × 10-28 kg
C) 9.0 × 10-36 kg
D) 5.4 × 10-32 kg
E) 3.2 × 10-27 kg
C
You might also like to view...
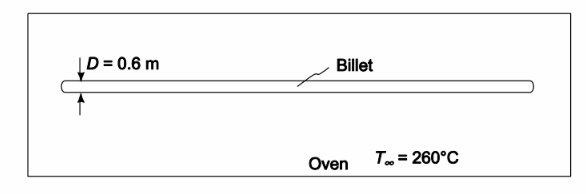
A long 0.6-m-OD 347 stainless steel (k = 14 W/(m K)) cylindrical billet at 16°C room temperature is placed in an oven where the temperature is 260°C. If the average heat transfer coefficient is 170 W/(m2 K), (a) estimate the time required for the center temperature to increase to 232°C by using the appropriate chart and (b) determine the instantaneous surface heat flux when the center temperature is 232°C.
GIVEN
• A long cylindrical billet placed in an oven
• Billet outside diameter = 0.6 m
• Thermal conductivity (k) = 14 W/(m K)
• Initial temperature (Ti) = 16°C
• Oven temperature (T?) = 260°C
• The average heat transfer coefficient h c= 170 W/(m2 K)
• Center temperature increases to 232°C
FIND
(a) The time required using the appropriate chart (b) The instantaneous surface heat fluxes when the center temperature is 232°C
ASSUMPTIONS
• Radial conduction only in billet
• Uniform and constant properties
SKETCH
A batter hits a home run in which the ball travels 110 m horizontally with no appreciable air resistance. If the ball left the bat at 50° above the horizontal just above ground level, how fast was it hit?
What will be an ideal response?
What potential difference is needed to accelerate a proton from rest to give it a wavelength of 2.00 × 10^–12 m?
a. 411 V b. 206 V c. 1650 V d. 617 V
The natural circulation of water—from ocean to air to ground to ocean and then back to the atmosphere—is called the
A) circle of life. B) hydrologic cycle. C) carbon cycle. D) rock cycle.