Which of the following statements is false?
A. According to the Gibbs phase rule, a mixture of saturated liquid water and saturated water vapor has one degree of freedom if the water is pure, but more degrees of freedom if there are other compounds dissolved in the water.
B. If the Gibbs phase rule says a system has one degree of freedom, and you specify a temperature for that system, you cannot also specify a pressure for that system—the pressure is constrained to a unique value.
C. A supercritical fluid cannot be converted into a saturated vapor by an isothermal process—the pressure and temperature would both have to change.
D. A supercritical fluid cannot be converted into a compressed liquid by an isobaric process—the pressure and temperature would both have to change.
E. If a one-component one-phase closed system undergoes a process that is isochoric and isobaric, it must also be isothermal.
A. Incorrect. This statement is true. There are two phases, so ? = 2. This cancels with the constant two and the equation reduces to F = C. Adding another chemical compound to the system—even if it dissolves in another—increases C and hence F.
B. Incorrect. This statement is true. The degrees of freedom of a system are the specifications you can make before all other intensive properties are constrained to unique values. If there is one degree of freedom and one intensive variable (Temperature) is specified, you have zero degrees of freedom left and the system and all intensive variables are completely “locked” in place.
C. Incorrect. This statement is true. Refer back to Figure 2-1. To get from the supercritical region to the vapor-liquid equilibrium line, both pressure and temperature have to decrease.
D. Correct. On Figure 2-1, if a temperature drop occurs to a supercritical fluid at constant pressure and the temperature falls below TC, the fluid reverts to a liquid.
E. Incorrect. This statement is true. For 1 phase and 1 component, ? = 1 and C = 1. The degrees of freedom are then F = 1 – 1 + 2 = 2. If V and T are both constant, then no other intensive system properties can change.
You might also like to view...
What is the disease triangle and why is it important for interiorscape technicians to understand it?
What will be an ideal response?
How can stereolithography printing help the manufacturing process?
What will be an ideal response?
What transportation infrastructure in northern England proved essential to the expansion of the Industrial Revolution?
a. railroads b. waterways c. roads d. bridges and overpasses
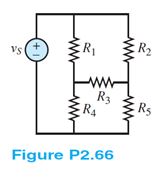
Find the equivalent resistance seen by the source in Figure P2.66. How many nodes are in the circuit?
Assume: R1 = 12 ? , R2 = 5 ? , R3 = 8 ? , R4 = 2 ? , and R5 = 4? .