If 1250mL of a gas is initially at temperature of 18°C and a pressure of 730mmHg, What would its volume be if the pressure were increased to 800mmHg and the temperature lowered to 10°C?
A. 625mL
B. 6337mL
C. 63.4mL
D. 1109mL
Answer: D
Mathematics
You might also like to view...
Solve the problem.Find the moment of inertia Iz of the rectangular solid of density 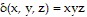 defined by
defined by 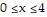 ,
, 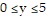 ,
, 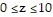 .
.
A. 352500 B. 312500 C. 290000 D. 102500
Mathematics
Find the square root.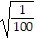
A. 100
B. 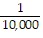
C. 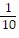
D. 10
Mathematics
Identify the property illustrated by the statement. Assume all variables represent real numbers.6 ? 1 = 6
A. Closure B. Distributive C. Identity D. Inverse
Mathematics
Use the Laws of Exponents to simplify. Write the answer with positive exponents. All variables are nonzero.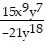
A. - 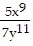
B. 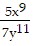
C. 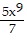
D. - 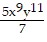
Mathematics