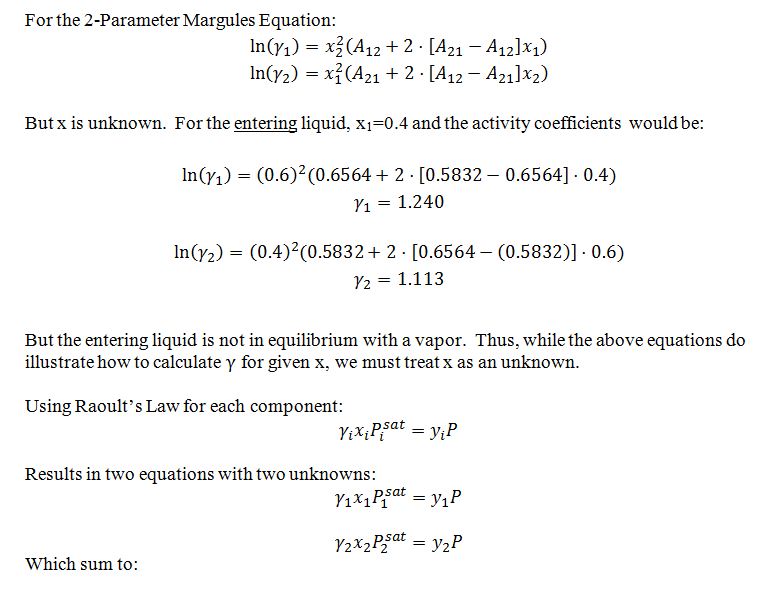
Eleven moles per second of a pentane (1) + benzene (2) system entering at 40 °C and 1500 mmHg is flashed at 40 °C and 500 mmHg. The mixture entering the flash evaporation unit is 40% by mole pentane. Estimate the molar flowrate and composition of the resulting phase(s) exiting the flash evaporation unit. Note the following information: • P1sat(40 C) = 867.07 mmHg • P2sat(40 C) = 182.64 mmHg • The liquid can be modeled by the 2-parameter Margules equation at this temperature, with the following parameters: A12 = 0.6564 A21= 0.5832
What will be an ideal response?
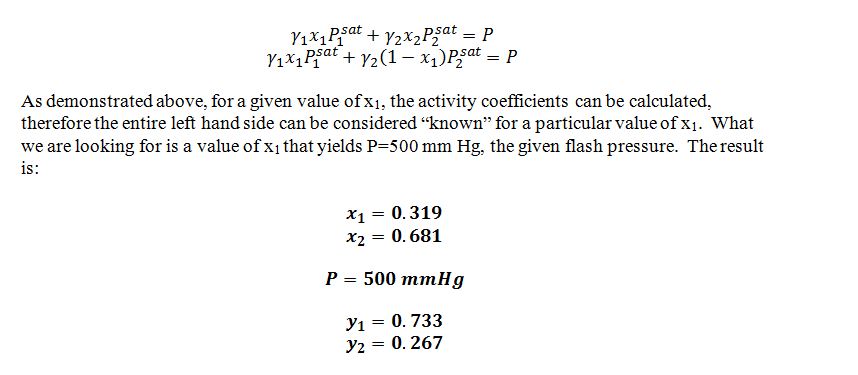
You might also like to view...
Higher-ordered living organisms are tremendously more complicated than machines governed by the laws of physics
Indicate whether the statement is true or false
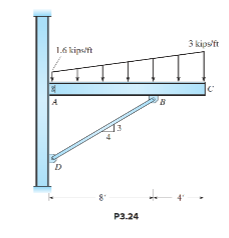
The clip angle connecting the beam’s web at A to the column may be assumed equivalent to a pin support. Assume member BD acts as an axially loaded pin-end compression strut. Compute the reactions at points A and D.
Headlights can be aimed by using ________
A) An aiming screen B) A bubble level C) Either A or B D) Neither A nor B
What do you call a device installed in an air distribution system, that is designed to close automatically upon detection of heat, to interrupt migratory airflow, and to restrict the passage of flame?
A. Fire Exhaust System B. Fire Damper C. Ceiling Damper D. Flame Spread System