You leave a hot cup of tea on a table. Where does the energy go?
What will be an ideal response?
The internal energy of the tea is transferred as heat to
the room because of a temperature diff erence between
the tea and room. The room’s temperature increases
imperceptibly.
You might also like to view...
This compartment is used in a revision history block to identify the location of the change on the print.
a. description b. location c. placement d. zone
Which of the following statements is true?
A rigid, adiabatic vessel is initially evacuated. 10 moles of liquid A and 10 moles of liquid B at 298.15 K are injected into the vessel, and this 20 moles of liquid is regarded as the “initial state” of the system. When the vessel reaches equilibrium, both a liquid and a vapor phase are present, and the entire equilibrium VLE mixture inside the vessel is regarded as the “final state” of the system. The reaction A+B?C+D occurs in the liquid phase, but not in the vapor phase. The equilibrium constant for the reaction is 0.4 and the reaction is exothermic. The vapor pressures are 0.12 MPa for compound A, 80kPa for compound B, 0.15 MPa for compound C and 70kPa for compound D. The vapor phase can be modeled as an ideal gas, but the liquid phase can NOT be modeled as an ideal solution.
A 5 ribbed serpentine belt should be tensioned at about? ________.
A) 45 to 60 lbs. B) 150 lbs. C) 75 to 100 lbs. D) None of these
A system has an impulse response 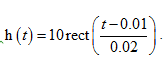 What is its null bandwidth?
What is its null bandwidth?
What will be an ideal response?