Originally 2.00 mol of gas are at standard temperature and pressure If the temperature changes to 47.0°C and the pressure decreases to half of what it was, how many liters do the two moles now occupy? (1 atm = 101 kPa, R = 8.31 J/mol ? K)
What will be an ideal response?
105 L
You might also like to view...
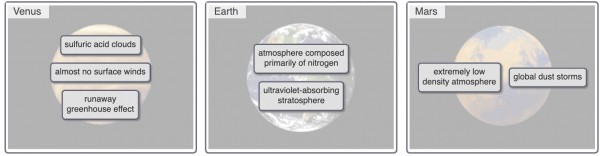
Listed following are characteristics of the atmospheres of Venus, Earth, and Mars. Match each atmospheric characteristic to the appropriate planet.
For every ton of coal burned, the approximate amount of CO2 injected into the atmosphere is
A) a few pounds. B) a ton. C) between 3 and 4 tons. D) half a ton. E) none, because the CO2 is recovered in solid form.
Four identical 1.5-mF capacitors are connected together electrically. What is the least possible capacitance of the combination?
a. 3.0E–4 F b. 2.5E–4 F c. 3.8E–4 F d. 1.5E–3 F e. 6.7E–4 F
A proton (m = 1.7 × 10^?27 kg, q = +1.6 × 10^?19 C) starts from rest at point A and has a speed of 40 km/s at point B. Only electric forces act on it during this motion. Determine the electric potential difference VB ? VA
a. +8.5 V b. ?8.5 V c. ?4.8 V d. +4.8 V e. ?17 V