Solve the problem.In a chemistry class, 7 liters of a 4% silver iodide solution must be mixed with a 10% solution to get a 6% solution. How many liters of the 10% solution are needed?
A. 3.5 L
B. 7.0 L
C. 2.5 L
D. 4.5 L
Answer: A
You might also like to view...
Use l'Hopital's Rule to evaluate the limit.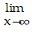
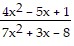
A. ?
B. 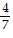
C. 1
D. 4
The magnitudes of the major earthquakes in 1999 are listed below. 1/19/99New Ireland, Papua New Guinea7.12/6/99Santa Cruz Islands, S.Pacific Sea7.43/4/99Celebes Sea, Indonesia7.24/5/99New Britain, Papua New Guinea7.54/8/99E.Russia/N.E.China border7.25/10/99New Britain, Papua New Guinea7.25/16/99New Britain, Papua New Guinea7.28/17/99Izmit region, western Turkey7.59/21/99Taiwan7.69/30/99Oaxaca, Mexico7.511/12/99Bolu Province, northwest Turkey7.3Find the mean, median, and mode of the magnitudes. Round to the nearest tenth.
A. mean 7.2; mode 7.1; median 7.4 B. mean 7.3; mode 7.3; median 7.3 C. mean 7.4; mode 7.3; median 7.3 D. mean 7.3; mode 7.2; median 7.3 E. mean 7.2; mode 7.2; median 7.3
Find the volume of the solid generated by revolving the shaded region about the given axis.About the y-axis

A.  ?
?
B. 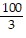 ?
?
C. 10?
D. 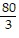 ?
?
Find the balance due on the maturity date of the note. Find the total amount of interest paid on the note. Use the United States Rule.Principal: $60,000Interest: 10%Time (days): 180Partial payment:$20,000 on day 60 $20,000 on day 120
A. $23,866.67; $3866.67 B. $21,652.18; $1652.18 C. $22,044.72; $2044.72 D. $24,022.42; $4022.42