Which of the following statements is true?
A. The ideal solution molar entropy for component i is equal to the pure component molar entropy of component i at the mixture temperature and pressure.
B. The ideal solution molar Gibbs free energy for component i is equal to the pure component molar Gibbs free energy of component i at the mixture temperature and pressure.
C. The ideal solution molar internal energy for component i is equal to the pure component molar internal energy of component iat the mixture temperature and pressure.
D. All of the above are true.
E. None of the above are true.
A. Incorrect. Review Table 9-2, especially the mixing properties column.
B. Incorrect. Review Table 9-2, especially the mixing properties column.
C. Correct. For an ideal solution there are no mixing properties for internal energy.
D. Incorrect. One or more statements are false.
E. Incorrect. At least one statement is true.
?
You might also like to view...
The circuit shown in the above figure contains two control relay coils. A normally closed contact, controlled by the opposite relay, is connected in series with each coil. When the switch is closed, which relay will turn on and which will be locked out of the circuit?
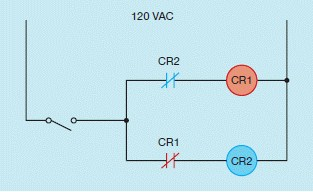
What will be an ideal response?
The National Electrical Code in Section 690.51 requires that ___________________ be clearly labeled on each module.
Fill in the blank(s) with the appropriate word(s).
Most milling machines have self-releasing tapers in their spindle sockets
Indicate whether the statement is true or false
Changes to the drawings will be noted in the _____
a. approval block b. legend c. revision block d. title block